Genetically encoded fluorescent voltage indicators: are we there yet?
- PMID: 29501950
- PMCID: PMC5984684
- DOI: 10.1016/j.conb.2018.02.006
Genetically encoded fluorescent voltage indicators: are we there yet?
Abstract
In order to understand how brain activity produces adaptive behavior we need large-scale, high-resolution recordings of neuronal activity. Fluorescent genetically encoded voltage indicators (GEVIs) offer the potential for these recordings to be performed chronically from targeted cells in a minimally invasive manner. As the number of GEVIs successfully tested for in vivo use grows, so has the number of open questions regarding the improvements that would facilitate broad adoption of this technology that surpasses mere 'proof of principle' studies. Our aim in this review is not to provide a status check of the current state of the field, as excellent publications covering this topic already exist. Here, we discuss specific questions regarding GEVI development and application that we think are crucial in achieving this goal.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest: None.
Figures

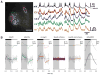
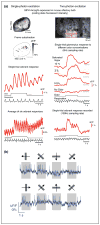
Similar articles
-
Optical voltage imaging in neurons: moving from technology development to practical tool.Nat Rev Neurosci. 2019 Dec;20(12):719-727. doi: 10.1038/s41583-019-0231-4. Epub 2019 Nov 8. Nat Rev Neurosci. 2019. PMID: 31705060 Review.
-
Genetically Encoded Voltage Indicators: Opportunities and Challenges.J Neurosci. 2016 Sep 28;36(39):9977-89. doi: 10.1523/JNEUROSCI.1095-16.2016. Epub 2016 Sep 28. J Neurosci. 2016. PMID: 27683896 Free PMC article. Review.
-
Genetically encoded voltage indicators for large scale cortical imaging come of age.Curr Opin Chem Biol. 2015 Aug;27:75-83. doi: 10.1016/j.cbpa.2015.06.006. Epub 2015 Jun 24. Curr Opin Chem Biol. 2015. PMID: 26115448 Review.
-
Imaging of Brain Slices with a Genetically Encoded Voltage Indicator.Methods Mol Biol. 2017;1563:73-84. doi: 10.1007/978-1-4939-6810-7_5. Methods Mol Biol. 2017. PMID: 28324602 Free PMC article.
-
The evolving capabilities of rhodopsin-based genetically encoded voltage indicators.Curr Opin Chem Biol. 2015 Aug;27:84-9. doi: 10.1016/j.cbpa.2015.05.006. Epub 2015 Jul 2. Curr Opin Chem Biol. 2015. PMID: 26143170 Free PMC article. Review.
Cited by
-
GRINtrode: a neural implant for simultaneous two-photon imaging and extracellular electrophysiology in freely moving animals.Neurophotonics. 2022 Oct;9(4):045009. doi: 10.1117/1.NPh.9.4.045009. Epub 2022 Dec 1. Neurophotonics. 2022. PMID: 36466189 Free PMC article.
-
Optogenetics for light control of biological systems.Nat Rev Methods Primers. 2022;2:55. doi: 10.1038/s43586-022-00136-4. Epub 2022 Jul 21. Nat Rev Methods Primers. 2022. PMID: 37933248 Free PMC article.
-
Optical voltage imaging in neurons: moving from technology development to practical tool.Nat Rev Neurosci. 2019 Dec;20(12):719-727. doi: 10.1038/s41583-019-0231-4. Epub 2019 Nov 8. Nat Rev Neurosci. 2019. PMID: 31705060 Review.
-
Fluorescence Imaging of Cell Membrane Potential: From Relative Changes to Absolute Values.Int J Mol Sci. 2023 Jan 26;24(3):2435. doi: 10.3390/ijms24032435. Int J Mol Sci. 2023. PMID: 36768759 Free PMC article. Review.
-
Chemical Targeting of Voltage Sensitive Dyes to Specific Cells and Molecules in the Brain.J Am Chem Soc. 2020 May 20;142(20):9285-9301. doi: 10.1021/jacs.0c00861. Epub 2020 May 12. J Am Chem Soc. 2020. PMID: 32395989 Free PMC article.
References
-
- Scanziani M, Häusser M. Electrophysiology in the age of light. Nature. 2009;461:930–939. - PubMed
-
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. - PubMed
-
- Spira ME, Hai A. Multi-electrode array technologies for neuroscience and cardiology. Nature Nanotech. 2013;8:83–94. - PubMed
-
- Zochowski M, Wachowiak M, Falk CX, Cohen LB, Lam YW, Antic SD, Zecevic D. Imaging membrane potential with voltage-sensitive dyes. Biol Bull. 2000;198:1–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

