Bacteria-like mesoporous silica-coated gold nanorods for positron emission tomography and photoacoustic imaging-guided chemo-photothermal combined therapy
- PMID: 29501970
- PMCID: PMC5880312
- DOI: 10.1016/j.biomaterials.2018.02.043
Bacteria-like mesoporous silica-coated gold nanorods for positron emission tomography and photoacoustic imaging-guided chemo-photothermal combined therapy
Abstract
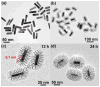
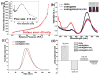
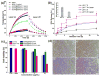
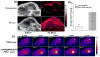
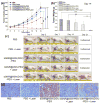
Mesoporous silica nanoshell (MSN) coating has been demonstrated as a versatile surface modification strategy for various kinds of inorganic functional nanoparticles, such as gold nanorods (GNRs), to achieve not only improved nanoparticle stability but also concomitant drug loading capability. However, limited drug loading capacity and low tumor accumulation rate in vivo are two major challenges for the biomedical applications of MSN-coated GNRs (GNR@MSN). In this study, by coating uniformly sized GNRs with MSN in an oil-water biphase reaction system, we have successfully synthesized a new bacteria-like GNR@MSN (i.e., bGNR@MSN) with a significantly enlarged pore size (4-8 nm) and surface area (470 m2/g). After PEGylation and highly efficient loading of doxorubicin (DOX, 40.9%, w/w), bGNR@MSN were used for positron emission tomography (PET, via facile and chelator-free 89Zr-labeling) and photoacoustic imaging-guided chemo-photothermal cancer therapy in vivo. PET imaging showed that 89Zr-labeled bGNR@MSN(DOX)-PEG can passively target to the 4T1 murine breast cancer-bearing mice with high efficiency (∼10 %ID/g), based on enhanced permeability and retention effect. Significantly enhanced chemo-photothermal combination therapy was also achieved due to excellent photothermal effect and near-infrared-light-triggered drug release by bGNR@MSN(DOX)-PEG at the tumor site. The promising results indicate great potential of bGNR@MSN-PEG nanoplatforms for future cancer diagnosis and therapy.
Keywords: Cancer; Chemo-photothermal therapy; Gold nanorods; Mesoporous silica nanoparticles; Positron emission tomography; Theranostics.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Notes
The authors declare no competing financial interest.
Figures


Similar articles
-
Gold nanoparticle-gated mesoporous silica as redox-triggered drug delivery for chemo-photothermal synergistic therapy.J Colloid Interface Sci. 2017 Dec 15;508:323-331. doi: 10.1016/j.jcis.2017.08.050. Epub 2017 Aug 16. J Colloid Interface Sci. 2017. PMID: 28843922
-
Mesoporous Carbon Nanoparticles as Multi-functional Carriers for Cancer Therapy Compared with Mesoporous Silica Nanoparticles.AAPS PharmSciTech. 2020 Jan 2;21(2):42. doi: 10.1208/s12249-019-1604-8. AAPS PharmSciTech. 2020. PMID: 31897882
-
Gold Nanorods-Based Smart Nanoplatforms for Synergic Thermotherapy and Chemotherapy of Tumor Metastasis.ACS Appl Mater Interfaces. 2019 Feb 27;11(8):7800-7811. doi: 10.1021/acsami.8b21784. Epub 2019 Feb 15. ACS Appl Mater Interfaces. 2019. PMID: 30720270
-
Radiolabeling Silica-Based Nanoparticles via Coordination Chemistry: Basic Principles, Strategies, and Applications.Acc Chem Res. 2018 Mar 20;51(3):778-788. doi: 10.1021/acs.accounts.7b00635. Epub 2018 Feb 28. Acc Chem Res. 2018. PMID: 29489335 Free PMC article. Review.
-
Mesoporous Silica-Encapsulated Gold Nanorods for Drug Delivery/Release and Two-Photon Excitation Fluorescence Imaging to Guide Synergistic Phototherapy and Chemotherapy.ACS Appl Bio Mater. 2023 Sep 18;6(9):3433-3440. doi: 10.1021/acsabm.3c00132. Epub 2023 Apr 21. ACS Appl Bio Mater. 2023. PMID: 37084245 Review.
Cited by
-
Optimising gold nanorods for photoacoustic imaging in vitro.Nanoscale Adv. 2019 Feb 12;1(4):1472-1481. doi: 10.1039/c8na00389k. eCollection 2019 Apr 9. Nanoscale Adv. 2019. PMID: 36132606 Free PMC article.
-
Gold Nanorods for LSPR Biosensing: Synthesis, Coating by Silica, and Bioanalytical Applications.Biosensors (Basel). 2020 Oct 17;10(10):146. doi: 10.3390/bios10100146. Biosensors (Basel). 2020. PMID: 33080925 Free PMC article. Review.
-
Natural and synthetic nanovectors for cancer therapy.Nanotheranostics. 2023 Mar 5;7(3):236-257. doi: 10.7150/ntno.77564. eCollection 2023. Nanotheranostics. 2023. PMID: 37064613 Free PMC article. Review.
-
Current update on nanoplatforms as therapeutic and diagnostic tools: A review for the materials used as nanotheranostics and imaging modalities.Asian J Pharm Sci. 2021 Jan;16(1):24-46. doi: 10.1016/j.ajps.2020.03.003. Epub 2020 May 12. Asian J Pharm Sci. 2021. PMID: 33613728 Free PMC article. Review.
-
Multifunctional phototheranostic nanomedicine for cancer imaging and treatment.Mater Today Bio. 2019 Nov 6;5:100035. doi: 10.1016/j.mtbio.2019.100035. eCollection 2020 Jan. Mater Today Bio. 2019. PMID: 32211603 Free PMC article. Review.
References
-
- Nguyen SC, Zhang Q, Manthiram K, Ye X, Lomont JP, Harris CB, Weller H, Alivisatos AP. Study of heat transfer dynamics from gold nanorods to the environment via time-resolved infrared spectroscopy. ACS Nano. 2016;10:2144–2151. - PubMed
-
- Tan SF, Anand U, Mirsaidov U. Interactions and attachment pathways between functionalized gold nanorods. ACS Nano. 2017;11:1633–1640. - PubMed
-
- Xu C, Yang D, Mei L, Lu B, Chen L, Li Q, Zhu H, Wang T. Encapsulating gold nanoparticles or nanorods in graphene oxide shells as a novel gene vector. ACS Appl Mater Interfaces. 2013;5:2715–2724. - PubMed
-
- Burrows ND, Lin W, Hinman JG, Dennison JM, Vartanian AM, Abadeer NS, Grzincic EM, Jacob LM, Li J, Murphy CJ. Surface chemistry of gold nanorods. Langmuir. 2016;32:9905–9921. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

