Safety and efficacy of the bumped kinase inhibitor BKI-1553 in pregnant sheep experimentally infected with Neospora caninum tachyzoites
- PMID: 29501973
- PMCID: PMC6114101
- DOI: 10.1016/j.ijpddr.2018.02.003
Safety and efficacy of the bumped kinase inhibitor BKI-1553 in pregnant sheep experimentally infected with Neospora caninum tachyzoites
Abstract
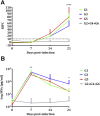
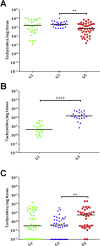
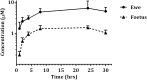
Neospora caninum is one of the main causes of abortion in cattle, and recent studies have highlighted its relevance as an abortifacient in small ruminants. Vaccines or drugs for the control of neosporosis are lacking. Bumped kinase inhibitors (BKIs), which are ATP-competitive inhibitors of calcium dependent protein kinase 1 (CDPK1), were shown to be highly efficacious against several apicomplexan parasites in vitro and in laboratory animal models. We here present the pharmacokinetics, safety and efficacy of BKI-1553 in pregnant ewes and foetuses using a pregnant sheep model of N. caninum infection. BKI-1553 showed exposure in pregnant ewes with trough concentrations of approximately 4 μM, and of 1 μM in foetuses. Subcutaneous BKI-1553 administration increased rectal temperatures shortly after treatment, and resulted in dermal nodules triggering a slight monocytosis after repeated doses at short intervals. BKI-1553 treatment decreased fever in infected pregnant ewes already after two applications, resulted in a 37-50% reduction in foetal mortality, and modulated immune responses; IFNγ levels were increased early after infection and IgG levels were reduced subsequently. N. caninum was abundantly found in placental tissues; however, parasite detection in foetal brain tissue decreased from 94% in the infected/untreated group to 69-71% in the treated groups. In summary, BKI-1553 confers partial protection against abortion in a ruminant experimental model of N. caninum infection during pregnancy. In addition, reduced parasite detection, parasite load and lesions in foetal brains were observed.
Keywords: BKI-1553; Neospora caninum; Pregnancy; Protein kinase inhibitor; Sheep; Treatment.
Copyright © 2018. Published by Elsevier Ltd.
Figures





References
-
- Almería S., Serrano-Pérez B., Darwich L., Domingo M., Mur-Novales R., Regidor-Cerrillo J., Cabezón O., Pérez-Maillo M., López-Helguera I., Fernández-Aguilar X. Foetal death in naive heifers inoculated with Neospora caninum isolate Nc-Spain7 at 110 days of pregnancy. Exp. Parasitol. 2016;168:62–69. - PubMed
-
- Álvarez-García G., Collantes-Fernández E., Costas E., Rebordosa X., Ortega-Mora L.M. Influence of age and purpose for testing on the cut-off selection of serological methods in bovine neosporosis. Vet. Res. 2003;34:341–352. - PubMed
-
- Arranz-Solís D., Benavides J., Regidor-Cerrillo J., Horcajo P., Castaño P., del Carmen Ferreras M., Jiménez-Pelayo L., Collantes-Fernández E., Ferre I., Hemphill A., Pérez V., Ortega-Mora L.M. Systemic and local immune responses in sheep after Neospora caninum experimental infection at early, mid and late gestation. Vet. Res. 2016;47:1–13. - PMC - PubMed
-
- Arranz-Solís D., Benavides J., Regidor-Cerrillo J., Fuertes M., Ferre I., Ferreras Mdel C., Collantes-Fernandez E., Hemphill A., Perez V., Ortega-Mora L.M. Influence of the gestational stage on the clinical course, lesional development and parasite distribution in experimental ovine neosporosis. Vet. Res. 2015;46:19. - PMC - PubMed
-
- Buxton D., Blewett D., Trees A., McColgan C., Finlayson J. Further studies in the use of monensin in the control of experimental ovine toxoplasmosis. J. Comp. Pathol. 1988;98:225–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

