Impact of acute otitis media clinical practice guidelines on antibiotic and analgesic prescriptions: a systematic review
- PMID: 29502073
- PMCID: PMC5965356
- DOI: 10.1136/archdischild-2017-314103
Impact of acute otitis media clinical practice guidelines on antibiotic and analgesic prescriptions: a systematic review
Abstract
Background: Clinical practice guidelines focusing on judicious use of antibiotics for childhood acute otitis media (AOM) have been introduced in many countries around the world.
Objective: To systematically review the effects of these guidelines on the prescription of antibiotics and analgesics for children with AOM.
Methods: Systematic searches of PubMed, Embase and Cochrane Library from inception to 6 June 2017 using broad search terms. Studies specifically aimed at evaluating the effects of introduction of national AOM practice guidelines on type of antibiotic and/or analgesic prescriptions were included, irrespective of design, setting or language. The Risk Of Bias In Non-randomized Studies of Interventions tool was used to assess risk of bias.
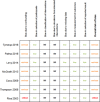
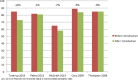
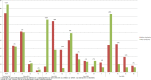
Results: Of 411 unique records retrieved, seven studies conducted in six different countries (France, Italy, Spain, Sweden, UK and USA (twice)) compared data before and after guideline introduction. All studies had an observational design, using longitudinal data of children aged under 15 years (n=200-4.6 million) from either routine care, insurance databases or electronic surveys. Risk of bias of all studies was judged serious to critical.Of the five studies reporting on antibiotic prescription rates, three showed a decline of 5%-12% up to 3 years after guideline introduction and two found no or negligible effect. In one US study, the initial 9% decline decreased to 5% after 4-6 years. The recommended first choice antibiotic was prescribed more frequently (9%-58% increase) after guideline introduction in four out of five studies reporting on this outcome. Analgesic prescription rates for AOM were reported in one US study and increased from 14% to 24% after guideline introduction.
Conclusion: Based upon what is published, the effects of introduction of national clinical practice guidelines on antibiotic and analgesic prescribing for children with AOM seem modest at the most.
Registration: PROSPERO: CRD42016050976.
Keywords: acute otitis Media; analgesics; antibiotics; aom; guidelines.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical