MyD88 and TLR4 Expression in Epithelial Ovarian Cancer
- PMID: 29502561
- PMCID: PMC5870793
- DOI: 10.1016/j.mayocp.2017.10.023
MyD88 and TLR4 Expression in Epithelial Ovarian Cancer
Abstract
Objective: To evaluate myeloid differentiation primary response gene 88 (MyD88) and Toll-like receptor 4 (TLR4) expression in relation to clinical features of epithelial ovarian cancer, histologic subtypes, and overall survival.
Patients and methods: We conducted centralized immunohistochemical staining, semi-quantitative scoring, and survival analysis in 5263 patients participating in the Ovarian Tumor Tissue Analysis consortium. Patients were diagnosed between January 1, 1978, and December 31, 2014, including 2865 high-grade serous ovarian carcinomas (HGSOCs), with more than 12,000 person-years of follow-up time. Tissue microarrays were stained for MyD88 and TLR4, and staining intensity was classified using a 2-tiered system for each marker (weak vs strong).
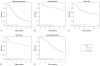
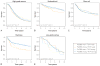
Results: Expression of MyD88 and TLR4 was similar in all histotypes except clear cell ovarian cancer, which showed reduced expression compared with other histotypes (P<.001 for both). In HGSOC, strong MyD88 expression was modestly associated with shortened overall survival (hazard ratio [HR], 1.13; 95% CI, 1.01-1.26; P=.04) but was also associated with advanced stage (P<.001). The expression of TLR4 was not associated with survival. In low-grade serous ovarian cancer (LGSOC), strong expression of both MyD88 and TLR4 was associated with favorable survival (HR [95% CI], 0.49 [0.29-0.84] and 0.44 [0.21-0.89], respectively; P=.009 and P=.02, respectively).
Conclusion: Results are consistent with an association between strong MyD88 staining and advanced stage and poorer survival in HGSOC and demonstrate correlation between strong MyD88 and TLR4 staining and improved survival in LGSOC, highlighting the biological differences between the 2 serous histotypes.
Copyright © 2017 Mayo Foundation for Medical Education and Research. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- U01 CA069417/CA/NCI NIH HHS/United States
- P50 CA159981/CA/NCI NIH HHS/United States
- P30 CA016056/CA/NCI NIH HHS/United States
- DH_/Department of Health/United Kingdom
- UL1 TR000124/TR/NCATS NIH HHS/United States
- C490/A16561/CRUK_/Cancer Research UK/United Kingdom
- UL1 TR001881/TR/NCATS NIH HHS/United States
- P50 CA136393/CA/NCI NIH HHS/United States
- M01 RR000056/RR/NCRR NIH HHS/United States
- R01 CA095023/CA/NCI NIH HHS/United States
- C490/A10119/CRUK_/Cancer Research UK/United Kingdom
- K07 CA080668/CA/NCI NIH HHS/United States
- C490/A10123/CRUK_/Cancer Research UK/United Kingdom
- R01 CA122443/CA/NCI NIH HHS/United States
- P30 CA015083/CA/NCI NIH HHS/United States
- K07 CA143047/CA/NCI NIH HHS/United States
- R01 CA160565/CA/NCI NIH HHS/United States
- 16561/CRUK_/Cancer Research UK/United Kingdom
- 10124/CRUK_/Cancer Research UK/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

