Human Papillomavirus Vaccine Coverage and Prevalence of Missed Opportunities for Vaccination in an Integrated Healthcare System
- PMID: 29502643
- PMCID: PMC6541918
- DOI: 10.1016/j.acap.2017.09.002
Human Papillomavirus Vaccine Coverage and Prevalence of Missed Opportunities for Vaccination in an Integrated Healthcare System
Abstract
Background: Human papillomavirus (HPV) vaccination has been recommended in the United States for female and male adolescents since 2006 and 2011, respectively. Coverage rates are lower than those for other adolescent vaccines. The objective of this study was to evaluate an assessment and feedback intervention designed to increase HPV vaccination coverage and quantify missed opportunities for HPV vaccine initiation at preventive care visits.
Methods: We examined changes in HPV vaccination coverage and missed opportunities within the adolescent (11-17 years) population at 9 Oregon-based Kaiser Permanente Northwest outpatient clinics after an assessment and feedback intervention. Quarterly coverage rates were calculated for the adolescent populations at the clinics, according to age group (11-12 and 13-17 years), sex, and department (Pediatrics and Family Medicine). Comparison coverage assessments were calculated at 3 nonintervention (control) clinics. Missed opportunities for HPV vaccine initiation, defined as preventive care visits in which a patient eligible for HPV dose 1 remained unvaccinated, were examined according to sex and age group.
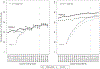
Results: An average of 29,021 adolescents were included in coverage assessments. Before the intervention, 1-dose and 3-dose quarterly coverage rates were increasing at intervention as well as at control clinics in both age groups. Postimplementation quarterly trends in 1-dose or 3-dose coverage did not differ significantly between intervention and control clinics for either age group. One-dose coverage rates among adolescents with Pediatrics providers were significantly higher than those with Family Medicine providers (56% vs 41% for 11- to 12-year-old and 82% vs 69% for 13- to 17-year-old girls; 55% vs 40% for 11- to 12-year-old and 78% vs 62% for 13- to 17-year-old boys).
Conclusions: No significant differences in HPV vaccine coverage were identified at intervention clinics. However, coverage rates were increasing before the start of the intervention and might have been influenced by ongoing health system best practices. HPV vaccine coverage rates varied significantly according to department, which could allow for targeted improvement opportunities.
Keywords: adolescent; human papillomavirus vaccine; vaccine coverage; vaccine initiation.
Copyright © 2017 Academic Pediatric Association. All rights reserved.
Conflict of interest statement
Ms Irving reports unrelated research support from Medimmune; Ms Groom reports unrelated research support from Merck, and Dr Naleway reports unrelated research support from Merck, Medimmune, and Pfizer. The remaining authors have no conflicts of interest to disclose.
Figures

Similar articles
-
A Learning Collaborative Model to Improve Human Papillomavirus Vaccination Rates in Primary Care.Acad Pediatr. 2018 Mar;18(2S):S46-S52. doi: 10.1016/j.acap.2018.01.003. Acad Pediatr. 2018. PMID: 29502638
-
Human papillomavirus vaccination coverage among adolescent boys and girls in the United States: A birth year cohort analysis of the National Immunization Survey-Teen, 2016-2022.Vaccine. 2025 Jan 12;44:126560. doi: 10.1016/j.vaccine.2024.126560. Epub 2024 Nov 30. Vaccine. 2025. PMID: 39615345 Free PMC article.
-
Early Outcomes of a Multilevel Human Papillomavirus Vaccination Pilot Intervention in Federally Qualified Health Centers.Acad Pediatr. 2018 Mar;18(2S):S79-S84. doi: 10.1016/j.acap.2017.11.001. Acad Pediatr. 2018. PMID: 29502642
-
US Health Care Clinicians' Knowledge, Attitudes, and Practices Regarding Human Papillomavirus Vaccination: A Qualitative Systematic Review.Acad Pediatr. 2018 Mar;18(2S):S53-S65. doi: 10.1016/j.acap.2017.10.007. Acad Pediatr. 2018. PMID: 29502639 Free PMC article.
-
Improving vaccination uptake among adolescents.Cochrane Database Syst Rev. 2020 Jan 17;1(1):CD011895. doi: 10.1002/14651858.CD011895.pub2. Cochrane Database Syst Rev. 2020. PMID: 31978259 Free PMC article.
Cited by
-
Missed opportunities for human papillomavirus vaccination at office visits during which influenza vaccine was administered: An AAP pediatric research in office settings (PROS) national primary care research network study.Vaccine. 2020 Jul 14;38(33):5105-5108. doi: 10.1016/j.vaccine.2020.05.090. Epub 2020 Jun 12. Vaccine. 2020. PMID: 32540274 Free PMC article.
-
A Systematic Review of Interventions to Improve HPV Vaccination Coverage.Vaccines (Basel). 2021 Jun 23;9(7):687. doi: 10.3390/vaccines9070687. Vaccines (Basel). 2021. PMID: 34201421 Free PMC article. Review.
-
Improving Human Papillomavirus Vaccination in the United States: Executive Summary.Acad Pediatr. 2018 Mar;18(2S):S1-S2. doi: 10.1016/j.acap.2018.01.009. Acad Pediatr. 2018. PMID: 29502626 Free PMC article. No abstract available.
-
Multilevel Targets for Promoting Pediatric HPV Vaccination: A Systematic Review of Parent-Centered, Provider-Centered, and Practice-Centered Interventions in HIC and LMIC Settings.Vaccines (Basel). 2025 Mar 11;13(3):300. doi: 10.3390/vaccines13030300. Vaccines (Basel). 2025. PMID: 40266195 Free PMC article. Review.
-
A Bundled, Practice-Based Intervention to Increase HPV Vaccination.Pediatrics. 2025 Feb 1;155(2):e2024068145. doi: 10.1542/peds.2024-068145. Pediatrics. 2025. PMID: 39756464 Free PMC article.
References
-
- Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years-United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:850–858. - PubMed
-
- Immunization Action Coalition. Tdap Booster Requirements for Secondary Schools. Available at: http://www.immunize.org/laws/tdap.asp. Accessed November 14, 2017.
-
- US Department of Health and Human Services. Healthy People 2020 Objectives: Immunization and Infectious Diseases. Washington, DC: USDHHS; 2014.
-
- Immunization Action Coalition. Meningococcal ACWY State Mandates for Elementary and Secondary Schools. Available at: http://www.immunize.org/laws/menin_sec.asp. Accessed November 14, 2017.
-
- National Cancer Institute. NCI-designated Cancer Centers Urge HPV Vaccination for the Prevention of Cancer. Bethesda, Md: National Cancer Institute; 2016.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

