Reducing Pericyte-Derived Scarring Promotes Recovery after Spinal Cord Injury
- PMID: 29502968
- PMCID: PMC5871719
- DOI: 10.1016/j.cell.2018.02.004
Reducing Pericyte-Derived Scarring Promotes Recovery after Spinal Cord Injury
Abstract
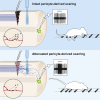
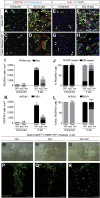
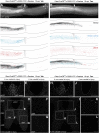
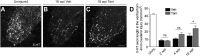
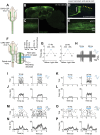
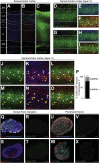
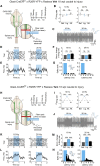
CNS injury often severs axons. Scar tissue that forms locally at the lesion site is thought to block axonal regeneration, resulting in permanent functional deficits. We report that inhibiting the generation of progeny by a subclass of pericytes led to decreased fibrosis and extracellular matrix deposition after spinal cord injury in mice. Regeneration of raphespinal and corticospinal tract axons was enhanced and sensorimotor function recovery improved following spinal cord injury in animals with attenuated pericyte-derived scarring. Using optogenetic stimulation, we demonstrate that regenerated corticospinal tract axons integrated into the local spinal cord circuitry below the lesion site. The number of regenerated axons correlated with improved sensorimotor function recovery. In conclusion, attenuation of pericyte-derived fibrosis represents a promising therapeutic approach to facilitate recovery following CNS injury.
Keywords: axon regeneration; fibrosis; optogenetics; pericyte; scar; sensorimotor functional recovery; spinal cord injury.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures








Comment in
-
To Scar or Not to Scar.Trends Mol Med. 2018 Jun;24(6):522-524. doi: 10.1016/j.molmed.2018.04.007. Epub 2018 May 2. Trends Mol Med. 2018. PMID: 29729835 Free PMC article.
References
-
- Arganda-Carreras I., Fernández-González R., Muñoz-Barrutia A., Ortiz-De-Solorzano C. 3D reconstruction of histological sections: Application to mammary gland tissue. Microsc. Res. Tech. 2010;73:1019–1029. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

