Statin dose reduction with complementary diet therapy: A pilot study of personalized medicine
- PMID: 29503145
- PMCID: PMC6001350
- DOI: 10.1016/j.molmet.2018.02.005
Statin dose reduction with complementary diet therapy: A pilot study of personalized medicine
Abstract
Objective: Statin intolerance, whether real or perceived, is a growing issue in clinical practice. Our aim was to evaluate the effects of reduced-dose statin therapy complemented with nutraceuticals.
Methods: First phase: Initially, 53 type 2 diabetic statin-treated patients received a supplementation with fish oil (1.7 g EPA + DHA/day), chocolate containing plant sterols (2.2 g/day), and green tea (two sachets/day) for 6 weeks. Second phase: "Good responders" to supplementation were identified after multivariate analysis (n = 10), and recruited for a pilot protocol of statin dose reduction. "Good responders" were then provided with supplementation for 12 weeks: standard statin therapy was kept during the first 6 weeks and reduced by 50% from weeks 6-12.
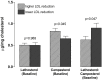
Results: First phase: After 6 weeks of supplementation, plasma LDL-C (-13.7% ± 3.7, P = .002) and C-reactive protein (-35.5% ± 5.9, P = .03) were reduced. Analysis of lathosterol and campesterol in plasma suggested that intensity of LDL-C reduction was influenced by cholesterol absorption rate rather than its synthesis. Second phase: no difference was observed for plasma lipids, inflammation, cholesterol efflux capacity, or HDL particles after statin dose reduction when compared to standard therapy.
Conclusions: Although limited by the small sample size, our study demonstrates the potential for a new therapeutic approach combining lower statin dose and specific dietary compounds. Further studies should elucidate "good responders" profile as a tool for personalized medicine. This may be particularly helpful in the many patients with or at risk for CVD who cannot tolerate high dose statin therapy.
Trial registration: ClinicalTrials.gov, NCT02732223.
Keywords: Atherosclerosis; Omega-3 fatty acids; Plant sterols; Polyphenols; Responders.
Copyright © 2018 The Authors. Published by Elsevier GmbH.. All rights reserved.
Figures
Similar articles
-
Dose-dependent effects of docosahexaenoic acid supplementation on blood lipids in statin-treated hyperlipidaemic subjects.Lipids. 2007 Mar;42(2):109-15. doi: 10.1007/s11745-006-3014-4. Epub 2007 Feb 8. Lipids. 2007. PMID: 17393216 Clinical Trial.
-
Long-term effects of ezetimibe-plus-statin therapy on low-density lipoprotein cholesterol levels as compared with double-dose statin therapy in patients with coronary artery disease.Atherosclerosis. 2012 Oct;224(2):454-6. doi: 10.1016/j.atherosclerosis.2012.07.036. Epub 2012 Aug 4. Atherosclerosis. 2012. PMID: 22892323 Clinical Trial.
-
Alterations of plant sterols, lathosterol, oxidative stress and inflammatory markers after the combination therapy of ezetimibe and statin drugs in type 2 diabetic patients.Obes Res Clin Pract. 2015 Jan-Feb;9(1):67-74. doi: 10.1016/j.orcp.2014.02.001. Epub 2014 Mar 27. Obes Res Clin Pract. 2015. PMID: 25660177
-
2017 Taiwan lipid guidelines for high risk patients.J Formos Med Assoc. 2017 Apr;116(4):217-248. doi: 10.1016/j.jfma.2016.11.013. Epub 2017 Feb 24. J Formos Med Assoc. 2017. PMID: 28242176 Review.
-
Management of statin-intolerant high-risk patients.Curr Vasc Pharmacol. 2010 Sep;8(5):632-7. doi: 10.2174/157016110792006932. Curr Vasc Pharmacol. 2010. PMID: 20507273 Review.
Cited by
-
Polyphenols and Fish Oils for Improving Metabolic Health: A Revision of the Recent Evidence for Their Combined Nutraceutical Effects.Molecules. 2021 Apr 22;26(9):2438. doi: 10.3390/molecules26092438. Molecules. 2021. PMID: 33922113 Free PMC article. Review.
-
New light on changes in the number and function of blood platelets stimulated by cocoa and its products.Front Pharmacol. 2024 Mar 12;15:1366076. doi: 10.3389/fphar.2024.1366076. eCollection 2024. Front Pharmacol. 2024. PMID: 38533262 Free PMC article. Review.
-
The role of tea in managing cardiovascular risk factors: potential benefits, mechanisms, and interventional strategies.Front Nutr. 2025 Apr 24;12:1530012. doi: 10.3389/fnut.2025.1530012. eCollection 2025. Front Nutr. 2025. PMID: 40342365 Free PMC article. Review.
-
The Role of n-3 Long Chain Polyunsaturated Fatty Acids in Cardiovascular Disease Prevention, and Interactions with Statins.Nutrients. 2018 Jun 15;10(6):775. doi: 10.3390/nu10060775. Nutrients. 2018. PMID: 29914111 Free PMC article. Review.
-
An insight to treat cardiovascular diseases through phytochemicals targeting PPAR-α.Mol Cell Biochem. 2024 Mar;479(3):707-732. doi: 10.1007/s11010-023-04755-7. Epub 2023 May 12. Mol Cell Biochem. 2024. PMID: 37171724 Review.
References
-
- Gotto A.M., Moon J.E. Pharmacotherapies for lipid modification: beyond the statins. Nature Reviews Cardiology. 2013;10:560–570. - PubMed
-
- Libby P., Ridker P.M., Hansson G.K. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. - PubMed
-
- Sabatine M.S., Giugliano R.P., Keech A.C., Honarpour N., Wiviott S.D., Murphy S.A. Evolocumab and clinical outcomes in patients with cardiovascular disease. New England Journal of Medicine. 2017;376:1713–1722. - PubMed
-
- Nicholls S.J., Puri R., Anderson T., Ballantyne C.M., Cho L., Kastelein J.J.P. Effect of evolocumab on progression of coronary disease in statin-treated patients. JAMA. 2016;316(22):2373. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials