Local Delivery of miR-21 Stabilizes Fibrous Caps in Vulnerable Atherosclerotic Lesions
- PMID: 29503197
- PMCID: PMC6080193
- DOI: 10.1016/j.ymthe.2018.01.011
Local Delivery of miR-21 Stabilizes Fibrous Caps in Vulnerable Atherosclerotic Lesions
Abstract
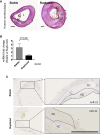
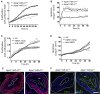
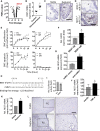
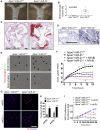
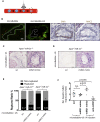
miRNAs are potential regulators of carotid artery stenosis and concordant vulnerable atherosclerotic plaques. Hence, we analyzed miRNA expression in laser captured micro-dissected fibrous caps of either ruptured or stable plaques (n = 10 each), discovering that miR-21 was significantly downregulated in unstable lesions. To functionally evaluate miR-21 in plaque vulnerability, miR-21 and miR-21/apolipoprotein-E double-deficient mice (Apoe-/-miR-21-/-) were assessed. miR-21-/- mice lacked sufficient smooth muscle cell proliferation in response to carotid ligation injury. When exposing Apoe-/-miR-21-/- mice to an inducible plaque rupture model, they presented with more atherothrombotic events (93%) compared with miR-21+/+Apoe-/- mice (57%). We discovered that smooth muscle cell fate in experimentally induced advanced lesions is steered via a REST-miR-21-REST feedback signaling pathway. Furthermore, Apoe-/-miR-21-/- mice presented with more pronounced atherosclerotic lesions, greater foam cell formation, and substantially higher levels of arterial macrophage infiltration. Local delivery of a miR-21 mimic using ultrasound-targeted microbubbles into carotid plaques rescued the vulnerable plaque rupture phenotype. In the present study, we identify miR-21 as a key modulator of pathologic processes in advanced atherosclerosis. Targeted, lesion site-specific overexpression of miR-21 can stabilize vulnerable plaques.
Keywords: atherosclerosis; microRNA; molecular medicine.
Copyright © 2018 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Figures



Comment in
-
MicroRNA-21 and the Vulnerability of Atherosclerotic Plaques.Mol Ther. 2018 Apr 4;26(4):938-940. doi: 10.1016/j.ymthe.2018.03.005. Epub 2018 Mar 21. Mol Ther. 2018. PMID: 29571964 Free PMC article. No abstract available.
References
-
- Mozaffarian D., Benjamin E.J., Go A.S., Arnett D.K., Blaha M.J., Cushman M., Das S.R., de Ferranti S., Després J.P., Fullerton H.J., Writing Group Members. American Heart Association Statistics Committee. Stroke Statistics Subcommittee Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e360. - PubMed
-
- Hajifathalian K., Ueda P., Lu Y., Woodward M., Ahmadvand A., Aguilar-Salinas C.A., Azizi F., Cifkova R., Di Cesare M., Eriksen L. A novel risk score to predict cardiovascular disease risk in national populations (Globorisk): a pooled analysis of prospective cohorts and health examination surveys. Lancet Diabetes Endocrinol. 2015;3:339–355. - PMC - PubMed
-
- Gupta A., Baradaran H., Schweitzer A.D., Kamel H., Pandya A., Delgado D., Dunning A., Mushlin A.I., Sanelli P.C. Carotid plaque MRI and stroke risk: a systematic review and meta-analysis. Stroke. 2013;44:3071–3077. - PubMed
-
- Howard D.P., van Lammeren G.W., Rothwell P.M., Redgrave J.N., Moll F.L., de Vries J.P., de Kleijn D.P., den Ruijter H.M., de Borst G.J., Pasterkamp G. Symptomatic carotid atherosclerotic disease: correlations between plaque composition and ipsilateral stroke risk. Stroke. 2015;46:182–189. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

