Visual acuity loss associated with excessive "dry macula" in exudative age-related macular degeneration
- PMID: 29503524
- PMCID: PMC5824750
- DOI: 10.2147/OPTH.S151999
Visual acuity loss associated with excessive "dry macula" in exudative age-related macular degeneration
Abstract
Purpose: To investigate the correlation between visual acuity and central macular thickness (CMT) and choroidal thickness (CCT) in patients with wet age-related macular degeneration (AMD).
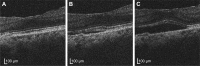
Methods: In this retrospective analysis, 14 eyes that received >10 ranibizumab injections (based on pro re nata [PRN] regimen) and maintained initial visual acuity gain were analyzed. The following 5 parameters were measured at the foveal center: CMT (distance from the inner limiting membrane [ILM] to Bruch's membrane); central retinal thickness (CRT; distance from the ILM to the inner limit of the retinal pigment epithelium or subretinal fluid [SRF]); SRF thickness (SRFT); pigment epithelium detachment thickness (PEDT); and CCT. The correlation between the logarithm of the minimum angle of resolution (logMAR) best-corrected visual acuity (BCVA) and the 5 parameters was examined with generalized estimating equations.
Results: CMT, CRT, and CCT were negatively correlated with logMAR BCVA (P=0.031, 0.023, and 0.036, respectively) when only CMT values less than the thickness that maximized visual acuity for each eye were used for the analysis. Each 100-μm reduction in CMT, CRT, or CCT improved logMAR BCVA by -0.1, -0.08, or -0.07, respectively. SRFT and PEDT were not correlated with BCVA. The median CMT that maximized the visual acuity was 230 μm.
Conclusion: Dry macula with CMT <230 μm was associated with temporary decrease in visual acuity in AMD patients whose visual acuity was maintained with PRN regimen.
Keywords: age-related macular degeneration; dry macula; intravitreal injections; ranibizumab; visual acuity.
Conflict of interest statement
Disclosure HT reports grants and personal fees from Novartis Pharmaceuticals, personal fees from Bayer Yakuhin, personal fees from Santen Pharmaceutical, personal fees from Kowa Pharmaceutical, and personal fees from Tochigi Prefectural Ophthalmologists Association, outside the submitted work. YI reports personal fees from Novartis Pharmaceuticals, Mitsubishi-Tanabe Pharmaceutical, and Tochigi Prefectural Ophthalmologists Association, outside the submitted work. YY reports personal fees from Novartis Pharmaceuticals, Bayer Healthcare, and Santen Pharmaceutical, outside the submitted work. HK reports personal fees from Santen Pharmaceutical, Mitsubishi-Tanabe Pharmaceutical, and Senju Pharmaceutical, outside the submitted work. XT, SI, SS, YA, and YF report no conflicts of interest in this work.
Figures

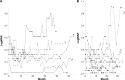
Similar articles
-
Comparison of one and three initial monthly intravitreal ranibizumab injection in patients with macular edema secondary to branch retinal vein occlusion.Int J Ophthalmol. 2018 Sep 18;11(9):1534-1538. doi: 10.18240/ijo.2018.09.17. eCollection 2018. Int J Ophthalmol. 2018. PMID: 30225230 Free PMC article.
-
Intravitreal Tissue Plasminogen Activator, Ranibizumab, and Gas Injection for Submacular Hemorrhage in Polypoidal Choroidal Vasculopathy.Ophthalmology. 2016 Jun;123(6):1278-86. doi: 10.1016/j.ophtha.2016.01.035. Epub 2016 Mar 2. Ophthalmology. 2016. PMID: 26949121
-
CHANGES IN CENTRAL CHOROIDAL THICKNESS AFTER TREATMENT OF DIABETIC MACULAR EDEMA WITH INTRAVITREAL BEVACIZUMAB CORRELATION WITH CENTRAL MACULAR THICKNESS AND BEST-CORRECTED VISUAL ACUITY.Retina. 2018 May;38(5):970-975. doi: 10.1097/IAE.0000000000001645. Retina. 2018. PMID: 28426622
-
Pigment epithelial detachment followed by retinal cystoid degeneration leads to vision loss in treatment of neovascular age-related macular degeneration.Ophthalmology. 2015 Apr;122(4):822-32. doi: 10.1016/j.ophtha.2014.11.017. Epub 2015 Jan 9. Ophthalmology. 2015. PMID: 25578255 Clinical Trial.
-
Five-year treatment outcomes following intravitreal ranibizumab injections for neovascular age-related macular degeneration in Japanese patients.Graefes Arch Clin Exp Ophthalmol. 2019 Jul;257(7):1411-1418. doi: 10.1007/s00417-019-04361-8. Epub 2019 May 22. Graefes Arch Clin Exp Ophthalmol. 2019. PMID: 31119425
Cited by
-
High frequency SD-OCT follow-up leading to up to biweekly intravitreal ranibizumab treatment in neovascular age-related macular degeneration.Sci Rep. 2021 Mar 25;11(1):6816. doi: 10.1038/s41598-021-86348-2. Sci Rep. 2021. PMID: 33767261 Free PMC article.
-
Retinal sensitivity above macular neovascularization under anti-VEGF therapy in exudative neovascular age-related macular degeneration.BMC Ophthalmol. 2025 Mar 12;25(1):123. doi: 10.1186/s12886-025-03946-8. BMC Ophthalmol. 2025. PMID: 40075283 Free PMC article.
References
-
- Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–e116. - PubMed
-
- Rosenfeld PJ, Brown DM, Heier JS, et al. MARINA Study Group Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. - PubMed
-
- Brown DM, Kaiser PK, Michels M, et al. ANCHOR Study Group Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–1444. - PubMed
-
- Chakravarthy U, Harding SP, Rogers CA, et al. IVAN study investigators Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet. 2013;382(9900):1258–1267. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

