Regulation of Osteoclast Differentiation by Cytokine Networks
- PMID: 29503739
- PMCID: PMC5833125
- DOI: 10.4110/in.2018.18.e8
Regulation of Osteoclast Differentiation by Cytokine Networks
Abstract
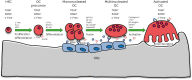
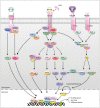
Cytokines play a pivotal role in maintaining bone homeostasis. Osteoclasts (OCs), the sole bone resorbing cells, are regulated by numerous cytokines. Macrophage colony-stimulating factor and receptor activator of NF-κB ligand play a central role in OC differentiation, which is also termed osteoclastogenesis. Osteoclastogenic cytokines, including tumor necrosis factor-α, IL-1, IL-6, IL-7, IL-8, IL-11, IL-15, IL-17, IL-23, and IL-34, promote OC differentiation, whereas anti-osteoclastogenic cytokines, including interferon (IFN)-α, IFN-β, IFN-γ, IL-3, IL-4, IL-10, IL-12, IL-27, and IL-33, downregulate OC differentiation. Therefore, dynamic regulation of osteoclastogenic and anti-osteoclastogenic cytokines is important in maintaining the balance between bone-resorbing OCs and bone-forming osteoblasts (OBs), which eventually affects bone integrity. This review outlines the osteoclastogenic and anti-osteoclastogenic properties of cytokines with regard to osteoimmunology, and summarizes our current understanding of the roles these cytokines play in osteoclastogenesis.
Keywords: Cytokines; Osteoclast differentiation factor; Osteoclastogenesis; Osteoimmunology.
Conflict of interest statement
Conflict of Interest: The authors declare no potential conflicts of interest.
Figures
References
-
- Rho J, Takami M, Choi Y. Osteoimmunology: interactions of the immune and skeletal systems. Mol Cells. 2004;17:1–9. - PubMed
-
- Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7:292–304. - PubMed
-
- Walsh MC, Kim N, Kadono Y, Rho J, Lee SY, Lorenzo J, Choi Y. Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol. 2006;24:33–63. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

