A Salutary Role of Reactive Oxygen Species in Intercellular Tunnel-Mediated Communication
- PMID: 29503816
- PMCID: PMC5821100
- DOI: 10.3389/fcell.2018.00002
A Salutary Role of Reactive Oxygen Species in Intercellular Tunnel-Mediated Communication
Abstract
The reactive oxygen species, generally labeled toxic due to high reactivity without target specificity, are gradually uncovered as signaling molecules involved in a myriad of biological processes. But one important feature of ROS roles in macromolecule movement has not caught attention until recent studies with technique advance and design elegance have shed lights on ROS signaling for intercellular and interorganelle communication. This review begins with the discussions of genetic and chemical studies on the regulation of symplastic dye movement through intercellular tunnels in plants (plasmodesmata), and focuses on the ROS regulatory mechanisms concerning macromolecule movement including small RNA-mediated gene silencing movement and protein shuttling between cells. Given the premise that intercellular tunnels (bridges) in mammalian cells are the key physical structures to sustain intercellular communication, movement of macromolecules and signals is efficiently facilitated by ROS-induced membrane protrusions formation, which is analogously applied to the interorganelle communication in plant cells. Although ROS regulatory differences between plant and mammalian cells exist, the basis for ROS-triggered conduit formation underlies a unifying conservative theme in multicellular organisms. These mechanisms may represent the evolutionary advances that have enabled multicellularity to gain the ability to generate and utilize ROS to govern material exchanges between individual cells in oxygenated environment.
Keywords: ROS; intercellular movement; interorganelle transport; macromolecule movement; membrane protrusions; multicellularization; plasmodesmata; tunneling nanotubes (TNTs).
Figures
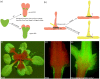
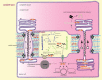

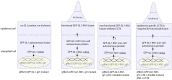
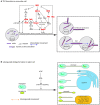

Similar articles
-
Plasmodesmata: intercellular tunnels facilitating transport of macromolecules in plants.Cell Tissue Res. 2013 Apr;352(1):49-58. doi: 10.1007/s00441-012-1550-1. Epub 2013 Feb 1. Cell Tissue Res. 2013. PMID: 23370600 Review.
-
Fine intercellular connections in development: TNTs, cytonemes, or intercellular bridges?Cell Stress. 2020 Jan 7;4(2):30-43. doi: 10.15698/cst2020.02.212. Cell Stress. 2020. PMID: 32043076 Free PMC article. Review.
-
New and old roles of plasmodesmata in immunity and parallels to tunneling nanotubes.Plant Sci. 2014 May;221-222:13-20. doi: 10.1016/j.plantsci.2014.01.006. Epub 2014 Jan 30. Plant Sci. 2014. PMID: 24656331 Free PMC article. Review.
-
Integrating Hormone- and Micromolecule-Mediated Signaling with Plasmodesmal Communication.Mol Plant. 2016 Jan 4;9(1):46-56. doi: 10.1016/j.molp.2015.08.015. Epub 2015 Sep 14. Mol Plant. 2016. PMID: 26384246 Review.
-
Exploring the role of lipids in intercellular conduits: breakthroughs in the pipeline.Front Plant Sci. 2013 Dec 10;4:504. doi: 10.3389/fpls.2013.00504. Front Plant Sci. 2013. PMID: 24368909 Free PMC article. Review.
Cited by
-
Peering into tunneling nanotubes-The path forward.EMBO J. 2021 Apr 15;40(8):e105789. doi: 10.15252/embj.2020105789. Epub 2021 Mar 1. EMBO J. 2021. PMID: 33646572 Free PMC article. Review.
-
Short Overview of ROS as Cell Function Regulators and Their Implications in Therapy Concepts.Cells. 2019 Jul 30;8(8):793. doi: 10.3390/cells8080793. Cells. 2019. PMID: 31366062 Free PMC article. Review.
-
Enzymatic Activity and Its Relationships with the Total Phenolic Content and Color Change in the High Hydrostatic Pressure-Assisted Curing of Vanilla Bean (Vanilla planifolia).Molecules. 2023 Nov 15;28(22):7606. doi: 10.3390/molecules28227606. Molecules. 2023. PMID: 38005328 Free PMC article.
-
Novel resources to investigate leaf plasmodesmata formation in C3 and C4 monocots.Plant J. 2024 Dec;120(5):2207-2225. doi: 10.1111/tpj.17113. Epub 2024 Nov 4. Plant J. 2024. PMID: 39494762 Free PMC article.
-
ROS Homeostasis in Abiotic Stress Tolerance in Plants.Int J Mol Sci. 2020 Jul 23;21(15):5208. doi: 10.3390/ijms21155208. Int J Mol Sci. 2020. PMID: 32717820 Free PMC article. Review.
References
-
- Agustí J., Gimeno J., Merelo P., Serrano R., Cercós M., Conesa A., et al. . (2012). Early gene expression events in the laminar abscission zone of abscission-promoted citrus leaves after a cycle of water stress/rehydration: involvement of CitbHLH1. J. Exp. Bot. 63, 6079–6091. 10.1093/jxb/ers270 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

