Quintupling Inhaled Glucocorticoids to Prevent Childhood Asthma Exacerbations
- PMID: 29504498
- PMCID: PMC5972517
- DOI: 10.1056/NEJMoa1710988
Quintupling Inhaled Glucocorticoids to Prevent Childhood Asthma Exacerbations
Abstract
Background: Asthma exacerbations occur frequently despite the regular use of asthma-controller therapies, such as inhaled glucocorticoids. Clinicians commonly increase the doses of inhaled glucocorticoids at early signs of loss of asthma control. However, data on the safety and efficacy of this strategy in children are limited.
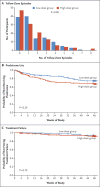
Methods: We studied 254 children, 5 to 11 years of age, who had mild-to-moderate persistent asthma and had had at least one asthma exacerbation treated with systemic glucocorticoids in the previous year. Children were treated for 48 weeks with maintenance low-dose inhaled glucocorticoids (fluticasone propionate at a dose of 44 μg per inhalation, two inhalations twice daily) and were randomly assigned to either continue the same dose (low-dose group) or use a quintupled dose (high-dose group; fluticasone at a dose of 220 μg per inhalation, two inhalations twice daily) for 7 days at the early signs of loss of asthma control ("yellow zone"). Treatment was provided in a double-blind fashion. The primary outcome was the rate of severe asthma exacerbations treated with systemic glucocorticoids.
Results: The rate of severe asthma exacerbations treated with systemic glucocorticoids did not differ significantly between groups (0.48 exacerbations per year in the high-dose group and 0.37 exacerbations per year in the low-dose group; relative rate, 1.3; 95% confidence interval, 0.8 to 2.1; P=0.30). The time to the first exacerbation, the rate of treatment failure, symptom scores, and albuterol use during yellow-zone episodes did not differ significantly between groups. The total glucocorticoid exposure was 16% higher in the high-dose group than in the low-dose group. The difference in linear growth between the high-dose group and the low-dose group was -0.23 cm per year (P=0.06).
Conclusions: In children with mild-to-moderate persistent asthma treated with daily inhaled glucocorticoids, quintupling the dose at the early signs of loss of asthma control did not reduce the rate of severe asthma exacerbations or improve other asthma outcomes and may be associated with diminished linear growth. (Funded by the National Heart, Lung, and Blood Institute; STICS ClinicalTrials.gov number, NCT02066129 .).
Conflict of interest statement
No other potential conflict of interest relevant to this article was reported.
Figures

Comment in
-
Escalating Inhaled Glucocorticoids to Prevent Asthma Exacerbations.N Engl J Med. 2018 Mar 8;378(10):950-952. doi: 10.1056/NEJMe1800152. Epub 2018 Mar 3. N Engl J Med. 2018. PMID: 29504500 No abstract available.
-
Inhaled Glucocorticoids in Asthma.N Engl J Med. 2018 May 24;378(21):2049-50. doi: 10.1056/NEJMc1804801. N Engl J Med. 2018. PMID: 29792405 No abstract available.
-
Inhaled Glucocorticoids in Asthma.N Engl J Med. 2018 May 24;378(21):2050. doi: 10.1056/NEJMc1804801. N Engl J Med. 2018. PMID: 29792406 No abstract available.
-
Inhaled Glucocorticoids in Asthma.N Engl J Med. 2018 May 24;378(21):2050-1. doi: 10.1056/NEJMc1804801. N Engl J Med. 2018. PMID: 29792407 No abstract available.
-
Quintupling inhaled fluticasone at first sign of exacerbation.J Pediatr. 2018 Sep;200:291-294. doi: 10.1016/j.jpeds.2018.07.014. J Pediatr. 2018. PMID: 30144926 No abstract available.
-
Kinder mit Asthma: ICS-Dosis nicht erhöhen.MMW Fortschr Med. 2018 Nov;160(20):38. doi: 10.1007/s15006-018-1170-0. MMW Fortschr Med. 2018. PMID: 30478563 German. No abstract available.
References
-
- Akinbami LJ, Moorman JE, Bailey C, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001-2010. NCHS Data Brief. 2012;94:1–8. - PubMed
-
- O’Byrne PM, Pedersen S, Lamm CJ, Tan WC, Busse WW. Severe exacerbations and decline in lung function in asthma. Am J Respir Crit Care Med. 2009;179:19–24. - PubMed
-
- Sorkness CA, Lemanske RF, Jr, Mauger DT, et al. Long-term comparison of 3 controller regimens for mild-moderate persistent childhood asthma: the Pediatric Asthma Controller Trial. J Allergy Clin Immunol. 2007;119:64–72. - PubMed
-
- Global Initiative for Asthma (GINA) Global strategy for asthma management and prevention. 2017 http://www.ginasthma.org/
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 HL098107/HL/NHLBI NIH HHS/United States
- U10 HL098075/HL/NHLBI NIH HHS/United States
- U10 HL098102/HL/NHLBI NIH HHS/United States
- U10 HL098115/HL/NHLBI NIH HHS/United States
- U10 HL098103/HL/NHLBI NIH HHS/United States
- UL1 TR002378/TR/NCATS NIH HHS/United States
- U10 HL098090/HL/NHLBI NIH HHS/United States
- U10 HL098098/HL/NHLBI NIH HHS/United States
- U10 HL064313/HL/NHLBI NIH HHS/United States
- K23 AI104780/AI/NIAID NIH HHS/United States
- P30 ES006694/ES/NIEHS NIH HHS/United States
- K23 AI106945/AI/NIAID NIH HHS/United States
- L40 AI107923/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
