Multimodal Neuroimaging in Alzheimer's Disease: Early Diagnosis, Physiopathological Mechanisms, and Impact of Lifestyle
- PMID: 29504542
- PMCID: PMC6004909
- DOI: 10.3233/JAD-179920
Multimodal Neuroimaging in Alzheimer's Disease: Early Diagnosis, Physiopathological Mechanisms, and Impact of Lifestyle
Abstract
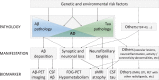
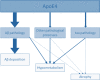
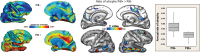
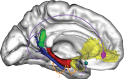
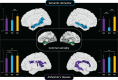
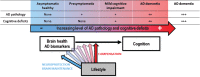
Over the last ten years, we have conducted research in Alzheimer's disease (AD) using multimodal neuroimaging techniques to improve diagnosis, further our understanding of the pathological mechanisms underlying the disease, and support the development of innovative non-pharmacological preventive strategies. Our works emphasized the interest of hippocampal subfield volumetry in early diagnosis and the need for further development in this field including optimization, standardization, and automatization of the techniques. Also, we conducted several studies in cognitively intact at-risk elderly (e.g., subjective cognitive decline patients and APOE4 carriers) to better identify biomarkers associated with increased risk of developing AD. Regarding the physiopathological mechanisms, specific multimodal neuroimaging techniques allowed us to highlight the relevance of diaschisis, the mismatch between neurodegeneration and local Aβ deposition and the regional variation in the mechanisms underlying structural or functional alterations. Further works integrating other biomarkers known to play a role in the physiopathology of AD (tau, TDP-43, inflammation, etc.) in a longitudinal design would be useful to get a comprehensive understanding of their relative role, sequence, and causal relationships. Our works also highlighted the relevance of functional connectivity in further understanding the specificity of cognitive deficits in AD and how connectivity differentially influences the propagation of the different AD biomarkers. Finally, we conducted several studies on the links between lifestyle factors and neuroimaging biomarkers to unravel mechanisms of reserve. Further efforts are needed to better understand which lifestyle factor, or combination of factors, impact on AD pathology, and when, to help translating our knowledge to training programs that might prevent or delay brain and cognitive changes leading to AD dementia.
Keywords: Aging; Alzheimer’s disease; FDG-PET; diagnosis; disconnection; lifestyle; meditation; multimodal neuroimaging; prevention; structural MRI.
Figures
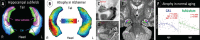

References
-
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 270–279. - PMC - PubMed
-
- Dubois B, Feldman HH, Jacova C, Cummings JL, Dekosky ST, Barberger-Gateau P, Delacourte A, Frisoni G, Fox NC, Galasko D, Gauthier S, Hampel H, Jicha GA, Meguro K, O’Brien J, Pasquier F, Robert P, Rossor M, Salloway S, Sarazin M, de Souza LC, Stern Y, Visser PJ, Scheltens P (2010) Revising the definition of Alzheimer’s disease: A new lexicon. Lancet Neurol 9, 1118–1127. - PubMed
-
- Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, Bakardjian H, Benali H, Bertram L, Blennow K, Broich K, Cavedo E, Crutch S, Dartigues J-F, Duyckaerts C, Epelbaum S, Frisoni GB, Gauthier S, Genthon R, Gouw AA, Habert M-O, Holtzman DM, Kivipelto M, Lista S, Molinuevo J-L, O’Bryant SE, Rabinovici GD, Rowe C, Salloway S, Schneider LS, Sperling R, Teichmann M, Carrillo MC, Cummings J, Jack CR, Proceedings of the Meeting of the International Working Group (IWG) and the American Alzheimer’s Association on “The Preclinical State of AD”, July 23, 2015, Washington DC, USA (2016) Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimers Dement 12, 292–323. - PMC - PubMed
-
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH (2011) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 263–269. - PMC - PubMed
-
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH (2011) Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 280–292. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

