Prospective study evaluating the use of nasal glucagon for the treatment of moderate to severe hypoglycaemia in adults with type 1 diabetes in a real-world setting
- PMID: 29504662
- PMCID: PMC5947579
- DOI: 10.1111/dom.13278
Prospective study evaluating the use of nasal glucagon for the treatment of moderate to severe hypoglycaemia in adults with type 1 diabetes in a real-world setting
Abstract
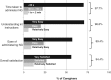
In the present multicentre, open-label, prospective, phase III study, we evaluated the real-world effectiveness and ease of use of nasal glucagon (NG) in the treatment of moderate/severe hypoglycaemic events (HEs) in adults with type 1 diabetes (T1D). Patients and caregivers were taught how to use NG (3 mg) to treat moderate/severe HEs, record the time taken to awaken or return to normal status, and measure blood glucose (BG) levels over time. Questionnaires were used to collect information about adverse events and ease of use of NG. In the efficacy analysis population, 69 patients experienced 157 HEs. In 95.7% patients, HEs resolved within 30 minutes of NG administration. In all the 12 severe HEs, patients awakened or returned to normal status within 15 minutes of NG administration without additional external medical help. Most caregivers reported that NG was easy to use. Most adverse events were local and of low to moderate severity. In this study, a single, 3-mg dose of NG demonstrated real-life effectiveness in treating moderate and severe HEs in adults with T1D. NG was well tolerated and easy to use.
Keywords: adults; hypoglycaemia; nasal glucagon; type 1 diabetes.
© 2018 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
E.R.S. is the Principal Investigator at University of Minnesota, Minneapolis. She also consults for Eli Lilly, Locemia, Zucara, Sanofi and Novo Nordisk and receives grants from Locemia and Eli Lilly. R.R‐L. is the Principal Investigator at Montreal Clinical Research Institute, Montreal, QC, Canada, and receives research grants from AMG, Astra‐Zeneca, Eli Lilly, Lexicon, Merck, NIH, Novo‐Nordisk and Sanofi‐Aventis. He is on the consulting/advisory panel of Abbott, Amgen, Astra‐Zeneca, Boehringer, Carlina Technologies, Eli Lilly, Janssen, Medtronic, Merck, Novo‐Nordisk, Roche, Sanofi‐Aventis and Takeda. G.M.T. is the Principal Investigator at McGill University Health Center, QC, Canada. J.R.C. is the Principal Investigator at Canadian Centre for Research on Diabetes, Smith Falls, ON, Canada. S.J.W. is the Principal Investigator at CHU de Québec‐Université Laval, Quebec City, QC, Canada. He receives research grants from Astra‐Zeneca, Eli Lilly, Novo‐Nordisk, Sanofi‐Aventis, Bayer, Novartis, Boehringer, Medtronic, Locemia and Diabetes Canada. G.F.G. is the Principal Investigator at Division of Community Endocrinology, Albany Medical College, Albany, New York. He receives research grants from Boehringer, Eli Lilly, Lexicon and Novo Nordisk and speaker honoraria from Boehringer, Dexcom, Janssen, Lilly, Merck & Novo Nordisk. V.C.W. is the Principal Investigator at University of Manitoba, Winnipeg, MB, Canada. He has been involved in clinical trials for Eli Lilly, Locemia, BMS, Astra Zeneca, Janssen, Merck, Novo Nordisk, Sanofi and he is on the advisory boards for Eli Lilly, Merck, Astra Zeneca, Novo Nordisk, Janssen and Sanofi. H.D., C.A.P. and D.C. are employees and stockholders of Locemia Solutions, which funded the study. X.M.Z. is an employee of and stockholder in Eli Lilly, Canada. S.P. is an employee of Eli Lilly, Bangalore, India. S.Z. is an employee of and stockholder in Eli Lilly and Company. C.B.G. is a former employee of and stockholder in Eli Lilly and Company. The authors report no other conflicts of interest.
Figures
References
-
- Haugstvedt A, Wentzel‐Larsen T, Graue M, Sovik O, Rokne B. Fear of hypoglycaemia in mothers and fathers of children with type 1 diabetes is associated with poor glycaemic control and parental emotional distress: a population‐based study. Diabet Med. 2010;27(1):72‐78. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical