Composition Dependence of Water Permeation Across Multicomponent Gel-Phase Bilayers
- PMID: 29504755
- PMCID: PMC6028149
- DOI: 10.1021/acs.jpcb.8b00747
Composition Dependence of Water Permeation Across Multicomponent Gel-Phase Bilayers
Abstract
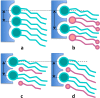
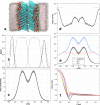
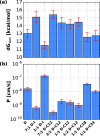
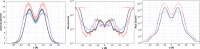
The permeability of multicomponent phospholipid bilayers in the gel phase is investigated via molecular dynamics simulation. The physical role of the different molecules is probed by comparing multiple mixed-component bilayers containing distearylphosphatidylcholine (DSPC) with varying amounts of either the emollient isostearyl isostearate or long-chain alcohol (dodecanol, octadecanol, or tetracosanol) molecules. Permeability is found to depend on both the tail packing density and hydrogen bonding between lipid headgroups and water. Whereas the addition of emollient or alcohol molecules to a gel-phase DSPC bilayer can increase the tail packing density, it also disturbed the hydrogen-bonding network, which in turn can increase interfacial water dynamics. These phenomena have opposing effects on bilayer permeability, which is found to depend on the balance between enhanced tail packing and decreased hydrogen bonding.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

