Disulfide-Bridged Peptides That Mediate Enantioselective Cycloadditions through Thiyl Radical Catalysis
- PMID: 29504763
- PMCID: PMC5963541
- DOI: 10.1021/acs.orglett.8b00364
Disulfide-Bridged Peptides That Mediate Enantioselective Cycloadditions through Thiyl Radical Catalysis
Abstract
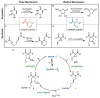
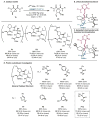
An enantioselective vinylcyclopropane ring-opening/cycloaddition cascade is described. The active thiyl radical catalysts are generated in situ via UV light-promoted homolysis of cystine-based dimers. Amide-functionalization of the peptide at the 4-proline position is essential for effective asymmetric induction. Stereochemical communication is dependent on steric interactions with this substituent that are enforced by H-bonding to the peptide backbone.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

