Role of CovR phosphorylation in gene transcription in Streptococcus mutans
- PMID: 29504927
- PMCID: PMC5982141
- DOI: 10.1099/mic.0.000641
Role of CovR phosphorylation in gene transcription in Streptococcus mutans
Abstract
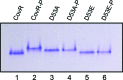
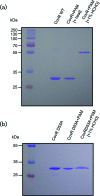
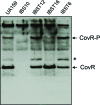
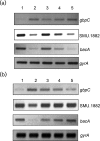
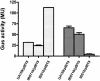
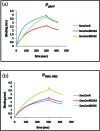
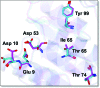
Streptococcus mutans, the primary aetiological agent of dental caries, is one of the major bacteria of the human oral cavity. The pathogenicity of this bacterium is attributed not only to the expression of virulence factors, but also to its ability to respond and adapt rapidly to the ever-changing conditions of the oral cavity. The two-component signal transduction system (TCS) CovR/S plays a crucial role in virulence and stress response in many streptococci. Surprisingly, in S. mutans the response regulator CovR appears to be an orphan, as the cognate sensor kinase, CovS, is absent in all the strains. We found that acetyl phosphate, an intracellular phosphodonor molecule known to act in signalling, might play a role in CovR phosphorylation in vivo. We also found that in vitro, upon phosphorylation by potassium phosphoramide (a high-energy phophodonor) CovR formed a dimer and showed altered electrophoretic mobility. As expected, we found that the conserved aspartic acid residue at position 53 (D53) was the site of phosphorylation, since neither phosphorylation nor dimerization was seen when an alanine-substituted CovR mutant (D53A) was used. Surprisingly, we found that the ability of CovR to act as a transcriptional regulator does not depend upon its phosphorylation status, since the D53A mutant behaved similarly to the wild-type protein in both in vivo and in vitro DNA-binding assays. This unique phosphorylation-mediated inhibition of CovR function in S. mutans sheds light on an unconventional mechanism of the signal transduction pathway.
Keywords: CovR; Streptococcus mutans; Two component regulator.
Conflict of interest statement
The authors declare there are no conflicts of interest.
Figures
Similar articles
-
CovR and VicRKX Regulate Transcription of the Collagen Binding Protein Cnm of Streptococcus mutans.J Bacteriol. 2018 Nov 6;200(23):e00141-18. doi: 10.1128/JB.00141-18. Print 2018 Dec 1. J Bacteriol. 2018. PMID: 30201780 Free PMC article.
-
Use of a Phosphorylation Site Mutant To Identify Distinct Modes of Gene Repression by the Control of Virulence Regulator (CovR) in Streptococcus pyogenes.J Bacteriol. 2017 Aug 22;199(18):e00835-16. doi: 10.1128/JB.00835-16. Print 2017 Sep 15. J Bacteriol. 2017. PMID: 28289082 Free PMC article.
-
CovR-controlled global regulation of gene expression in Streptococcus mutans.PLoS One. 2011;6(5):e20127. doi: 10.1371/journal.pone.0020127. Epub 2011 May 31. PLoS One. 2011. PMID: 21655290 Free PMC article.
-
The VicRK Two-Component System Regulates Streptococcus mutans Virulence.Curr Issues Mol Biol. 2019;32:167-200. doi: 10.21775/cimb.032.167. Epub 2019 Jun 5. Curr Issues Mol Biol. 2019. PMID: 31166172 Review.
-
ComDE Two-component Signal Transduction Systems in Oral Streptococci: Structure and Function.Curr Issues Mol Biol. 2019;32:201-258. doi: 10.21775/cimb.032.201. Epub 2019 Jun 5. Curr Issues Mol Biol. 2019. PMID: 31166173 Review.
Cited by
-
CovS inactivation reduces CovR promoter binding at diverse virulence factor encoding genes in group A Streptococcus.PLoS Pathog. 2022 Feb 18;18(2):e1010341. doi: 10.1371/journal.ppat.1010341. eCollection 2022 Feb. PLoS Pathog. 2022. PMID: 35180278 Free PMC article.
-
Group A Streptococcal asparagine metabolism regulates bacterial virulence.EMBO Rep. 2025 May;26(10):2767-2791. doi: 10.1038/s44319-025-00447-z. Epub 2025 Apr 14. EMBO Rep. 2025. PMID: 40229432 Free PMC article.
-
A novel insight into ComE-mediated activation of gene expression in Streptococcus mutans.Microbiol Spectr. 2025 Aug 5;13(8):e0147725. doi: 10.1128/spectrum.01477-25. Epub 2025 Jul 7. Microbiol Spectr. 2025. PMID: 40621915 Free PMC article.
-
Two-Component Signal Transduction Systems in the Human Pathogen Streptococcus agalactiae.Infect Immun. 2020 Jun 22;88(7):e00931-19. doi: 10.1128/IAI.00931-19. Print 2020 Jun 22. Infect Immun. 2020. PMID: 31988177 Free PMC article. Review.
-
Identification of Hanks-Type Kinase PknB-Specific Targets in the Streptococcus thermophilus Phosphoproteome.Front Microbiol. 2019 Jun 19;10:1329. doi: 10.3389/fmicb.2019.01329. eCollection 2019. Front Microbiol. 2019. PMID: 31275266 Free PMC article.
References
-
- Carlsson J, Hamilton I. Textbook of Clinical Cariology. 1994. Metabolic activity of oral bacteria; pp. 71–88.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

