PD-L1 in tumor microenvironment mediates resistance to oncolytic immunotherapy
- PMID: 29504948
- PMCID: PMC5873884
- DOI: 10.1172/JCI98047
PD-L1 in tumor microenvironment mediates resistance to oncolytic immunotherapy
Erratum in
-
PD-L1 in tumor microenvironment mediates resistance to oncolytic immunotherapy.J Clin Invest. 2018 Nov 1;128(11):5184. doi: 10.1172/JCI125039. Epub 2018 Oct 2. J Clin Invest. 2018. PMID: 30277478 Free PMC article. No abstract available.
Abstract
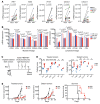
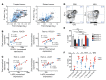
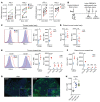
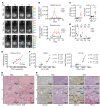
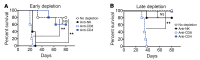
Intralesional therapy with oncolytic viruses (OVs) leads to the activation of local and systemic immune pathways, which may present targets for further combinatorial therapies. Here, we used human tumor histocultures as well as syngeneic tumor models treated with Newcastle disease virus (NDV) to identify a range of immune targets upregulated with OV treatment. Despite tumor infiltration of effector T lymphocytes in response to NDV, there was ongoing inhibition through programmed death ligand 1 (PD-L1), acting as a mechanism of early and late adaptive immune resistance to the type I IFN response and T cell infiltration, respectively. Systemic therapeutic targeting of programmed cell death receptor 1 (PD-1) or PD-L1 in combination with intratumoral NDV resulted in the rejection of both treated and distant tumors. These findings have implications for the timing of PD-1/PD-L1 blockade in conjunction with OV therapy and highlight the importance of understanding the adaptive mechanisms of immune resistance to specific OVs for the rational design of combinatorial approaches using these agents.
Keywords: Gene therapy; Immunology; Immunotherapy; Oncology; T cells.
Conflict of interest statement
Figures



Comment in
-
Unleashing the therapeutic potential of oncolytic viruses.J Clin Invest. 2018 Apr 2;128(4):1258-1260. doi: 10.1172/JCI120303. Epub 2018 Mar 5. J Clin Invest. 2018. PMID: 29504947 Free PMC article.
References
-
- Kaufman HL, Kim DW, DeRaffele G, Mitcham J, Coffin RS, Kim-Schulze S. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann Surg Oncol. 2010;17(3):718–730. doi: 10.1245/s10434-009-0809-6. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

