Parenteral Protein Decision Support System Improves Protein Delivery in Preterm Infants: A Randomized Clinical Trial
- PMID: 29505147
- PMCID: PMC5841609
- DOI: 10.1002/jpen.1034
Parenteral Protein Decision Support System Improves Protein Delivery in Preterm Infants: A Randomized Clinical Trial
Abstract
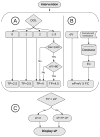
Background: Management of neonatal parenteral protein intake for preterm infants is challenging and requires daily modifications of the dose to account for the infant's postnatal age, birth weight, current weight, and the volume and protein concentration of concurrent enteral nutrition. The objective of this study was to create and evaluate the Parenteral Protein Calculator (PPC), a clinical decision support system to improve the accuracy of protein intake for preterm infants who require parenteral nutrition (PN).
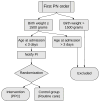
Materials and methods: We integrated the PPC into the computerized provider order entry system and tested it in a randomized controlled trial (routine or PPC). Infants were eligible if they were ≤3 days old, had a birth weight ≤1500 g, and had no inborn error of metabolism. The primary outcome was the appropriate total protein intake, defined as target protein dose ±0.5 g/kg.
Results: We randomly allocated 42 infants for 221 PN days in the control group and 211 in the PPC group. Total protein intake in the PPC group was more accurate as compared with the control group (appropriate protein dosing: odds ratio = 5.8; 95% CI, 2.7-12.4). Absolute deviation from protein target was 0.41 g/kg (0.24-0.58) lower in the PPC group.
Conclusion: The PPC improved appropriate protein dosing for premature infants receiving PN. Further studies are needed to test whether clinical decision support systems will reduce uremia and improve growth and to replicate similar findings in the cases of other PN nutrients.
Keywords: clinical decision support system; medication dosing; parenteral nutrition; protein intake; uremia.
© 2017 American Society for Parenteral and Enteral Nutrition.
Conflict of interest statement
Christoph U. Lehmann, MD received royalties from Springer Verlag for the book ‘Pediatric Informatics’. He serves as the Medical Director of the Child Health Informatics Center at the American Academy of Pediatrics, editor-in-chief of the journal Applied Clinical Informatics, and as President Elect of the International Medical Informatics Association.
The other authors have indicated they have no financial relationships relevant to this article to disclose.
Figures

References
-
- American Academy of Pediatrics, Committee on Nutrition. Nutritional needs of low-birth-weight infants. Pediatrics. 1977;60:519–5. - PubMed
-
- Lemons JA, Bauer CR, Oh W, et al. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network. Pediatrics. 2001;107(1):e1. - PubMed
-
- Ehrenkranz RA, Younes N, Lemons JA, et al. Longitudinal growth of hospitalized very low birth weight infants. Pediatrics. 1999;104:280–289. - PubMed
-
- Ziegler EE, Thureen PJ, Carlson SJ. Aggressive nutrition of the very low birth weight infant. Clinical Perinatology. 2002;29:225–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

