Identification of binding sites contributing to volatile anesthetic effects on GABA type A receptors
- PMID: 29505303
- PMCID: PMC6044061
- DOI: 10.1096/fj.201701347R
Identification of binding sites contributing to volatile anesthetic effects on GABA type A receptors
Abstract
Most general anesthetics enhance GABA type A (GABAA) receptor activity at clinically relevant concentrations. Sites of action of volatile anesthetics on the GABAA receptor remain unknown, whereas sites of action of many intravenous anesthetics have been identified in GABAA receptors by using photolabeling. Here, we used photoactivatable analogs of isoflurane (AziISO) and sevoflurane (AziSEVO) to locate their sites on α1β3γ2L and α1β3 GABAA receptors. As with isoflurane and sevoflurane, AziISO and AziSEVO enhanced the currents elicited by GABA. AziISO and AziSEVO each labeled 10 residues in α1β3 receptors and 9 and 8 residues, respectively, in α1β3γ2L receptors. Photolabeled residues were concentrated in transmembrane domains and located in either subunit interfaces or in the interface between the extracellular domain and the transmembrane domain. The majority of these transmembrane residues were protected from photolabeling with the addition of excess parent anesthetic, which indicated specificity. Binding sites were primarily located within α+/β- and β+/α- subunit interfaces, but residues in the α+/γ- interface were also identified, which provided a basis for differential receptor subtype sensitivity. Isoflurane and sevoflurane did not always share binding sites, which suggests an unexpected degree of selectivity.-Woll, K. A., Zhou, X., Bhanu, N. V., Garcia, B. A., Covarrubias, M., Miller, K. W., Eckenhoff, R. G. Identification of binding sites contributing to volatile anesthetic effects on GABA type A receptors.
Keywords: crosslinking; isoflurane; photoaffinity labeling; sevoflurane.
Conflict of interest statement
This work was supported by U.S. National Institutes of Health Grants P01-GM55876, GM107117, GM110174, P01–GM58448 (National Institute of General Medical Sciences), and AI118891 (Department of Defense); and by the National Science Foundation Graduate Research Fellowship Program (DGE-1321851). The authors declare no conflicts of interest.
Figures





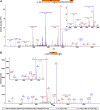
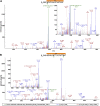





Similar articles
-
The actions of sevoflurane and desflurane on the gamma-aminobutyric acid receptor type A: effects of TM2 mutations in the alpha and beta subunits.Anesthesiology. 2003 Sep;99(3):678-84. doi: 10.1097/00000542-200309000-00024. Anesthesiology. 2003. PMID: 12960553
-
Specificity of intersubunit general anesthetic-binding sites in the transmembrane domain of the human α1β3γ2 γ-aminobutyric acid type A (GABAA) receptor.J Biol Chem. 2013 Jul 5;288(27):19343-57. doi: 10.1074/jbc.M113.479725. Epub 2013 May 15. J Biol Chem. 2013. PMID: 23677991 Free PMC article.
-
Inhibitable photolabeling by neurosteroid diazirine analog in the β3-Subunit of human hetereopentameric type A GABA receptors.Eur J Med Chem. 2019 Jan 15;162:810-824. doi: 10.1016/j.ejmech.2018.11.019. Epub 2018 Nov 19. Eur J Med Chem. 2019. PMID: 30544077 Free PMC article.
-
GABA(A) receptors as molecular targets of general anesthetics: identification of binding sites provides clues to allosteric modulation.Can J Anaesth. 2011 Feb;58(2):206-15. doi: 10.1007/s12630-010-9429-7. Epub 2010 Dec 31. Can J Anaesth. 2011. PMID: 21194017 Free PMC article. Review.
-
The effects of volatile anesthetics on synaptic and extrasynaptic GABA-induced neurotransmission.Brain Res Bull. 2013 Apr;93:69-79. doi: 10.1016/j.brainresbull.2012.08.001. Epub 2012 Aug 17. Brain Res Bull. 2013. PMID: 22925739 Review.
Cited by
-
Astrocyte morphological remodeling regulates consciousness state transitions induced by inhaled general anesthesia.Mol Psychiatry. 2025 Sep;30(9):4006-4022. doi: 10.1038/s41380-025-02978-2. Epub 2025 Apr 1. Mol Psychiatry. 2025. PMID: 40169801
-
The Biology of General Anesthesia from Paramecium to Primate.Curr Biol. 2019 Nov 18;29(22):R1199-R1210. doi: 10.1016/j.cub.2019.09.071. Curr Biol. 2019. PMID: 31743680 Free PMC article. Review.
-
The antinociceptive effect of artemisinin on the inflammatory pain and role of GABAergic and opioidergic systems.Korean J Pain. 2019 Jul 1;32(3):160-167. doi: 10.3344/kjp.2019.32.3.160. Korean J Pain. 2019. PMID: 31257824 Free PMC article.
-
Open-channel blocking action of volatile anaesthetics desflurane and sevoflurane on human voltage-gated Kv 1.5 channel.Br J Pharmacol. 2020 Aug;177(16):3811-3827. doi: 10.1111/bph.15105. Epub 2020 Jun 30. Br J Pharmacol. 2020. PMID: 32436224 Free PMC article.
-
GABAA Receptors in Astrocytes Are Targets for Commonly Used Intravenous and Inhalational General Anesthetic Drugs.Front Aging Neurosci. 2022 Jan 11;13:802582. doi: 10.3389/fnagi.2021.802582. eCollection 2021. Front Aging Neurosci. 2022. PMID: 35087395 Free PMC article.
References
-
- Curtis D. R., Duggan A. W., Felix D., Johnston G. A. (1971) Bicuculline, an antagonist of GABA and synaptic inhibition in the spinal cord of the cat. Brain Res. 32, 69–96 - PubMed
-
- Mihic S. J., McQuilkin S. J., Eger E. I., II, Ionescu P., Harris R. A. (1994) Potentiation of gamma-aminobutyric acid type A receptor-mediated chloride currents by novel halogenated compounds correlates with their abilities to induce general anesthesia. Mol. Pharmacol. 46, 851–857 - PubMed
-
- Brown C. L., Martin I. L. (1983) Photoaffinity labelling of the benzodiazepine receptor cannot be used to predict ligand efficacy. Neurosci. Lett. 35, 37–40 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

