A FRET-based biosensor for measuring Gα13 activation in single cells
- PMID: 29505611
- PMCID: PMC5837189
- DOI: 10.1371/journal.pone.0193705
A FRET-based biosensor for measuring Gα13 activation in single cells
Erratum in
-
Correction: A FRET-based biosensor for measuring Gα13 activation in single cells.PLoS One. 2018 Apr 4;13(4):e0195649. doi: 10.1371/journal.pone.0195649. eCollection 2018. PLoS One. 2018. PMID: 29617442 Free PMC article.
Abstract
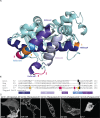
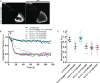
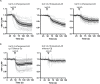
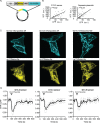
Förster Resonance Energy Transfer (FRET) provides a way to directly observe the activation of heterotrimeric G-proteins by G-protein coupled receptors (GPCRs). To this end, FRET based biosensors are made, employing heterotrimeric G-protein subunits tagged with fluorescent proteins. These FRET based biosensors complement existing, indirect, ways to observe GPCR activation. Here we report on the insertion of mTurquoise2 at several sites in the human Gα13 subunit, aiming to develop a FRET-based Gα13 activation biosensor. Three fluorescently tagged Gα13 variants were found to be functional based on i) plasma membrane localization and ii) ability to recruit p115-RhoGEF upon activation of the LPA2 receptor. The tagged Gα13 subunits were used as FRET donor and combined with cp173Venus fused to the Gγ2 subunit, as the acceptor. We constructed Gα13 biosensors by generating a single plasmid that produces Gα13-mTurquoise2, Gβ1 and cp173Venus-Gγ2. The Gα13 activation biosensors showed a rapid and robust response when used in primary human endothelial cells that were exposed to thrombin, triggering endogenous protease activated receptors (PARs). This response was efficiently inhibited by the RGS domain of p115-RhoGEF and from the biosensor data we inferred that this is due to GAP activity. Finally, we demonstrated that the Gα13 sensor can be used to dissect heterotrimeric G-protein coupling efficiency in single living cells. We conclude that the Gα13 biosensor is a valuable tool for live-cell measurements that probe spatiotemporal aspects of Gα13 activation.
Conflict of interest statement
Figures

Similar articles
-
A New Generation of FRET Sensors for Robust Measurement of Gαi1, Gαi2 and Gαi3 Activation Kinetics in Single Cells.PLoS One. 2016 Jan 22;11(1):e0146789. doi: 10.1371/journal.pone.0146789. eCollection 2016. PLoS One. 2016. PMID: 26799488 Free PMC article.
-
Distinct regions of Galpha13 participate in its regulatory interactions with RGS homology domain-containing RhoGEFs.Cell Signal. 2007 Aug;19(8):1681-9. doi: 10.1016/j.cellsig.2007.03.004. Epub 2007 Mar 15. Cell Signal. 2007. PMID: 17449226
-
Critical role of lysine 204 in switch I region of Galpha13 for regulation of p115RhoGEF and leukemia-associated RhoGEF.Mol Pharmacol. 2004 Oct;66(4):1029-34. doi: 10.1124/mol.104.002287. Epub 2004 Jul 16. Mol Pharmacol. 2004. PMID: 15258251
-
GPCR-Gα13 Involvement in Mitochondrial Function, Oxidative Stress, and Prostate Cancer.Int J Mol Sci. 2024 Jun 28;25(13):7162. doi: 10.3390/ijms25137162. Int J Mol Sci. 2024. PMID: 39000269 Free PMC article. Review.
-
Single-cell analysis of G-protein signal transduction.J Biol Chem. 2015 Mar 13;290(11):6681-8. doi: 10.1074/jbc.R114.616391. Epub 2015 Jan 20. J Biol Chem. 2015. PMID: 25605723 Free PMC article. Review.
Cited by
-
Rapid flow-induced activation of Gαq/11 is independent of Piezo1 activation.Am J Physiol Cell Physiol. 2019 May 1;316(5):C741-C752. doi: 10.1152/ajpcell.00215.2018. Epub 2019 Feb 27. Am J Physiol Cell Physiol. 2019. PMID: 30811222 Free PMC article.
-
Angiotensin receptors and α1B-adrenergic receptors regulate native IK(ACh) and phosphorylation-deficient GIRK4 (S418A) channels through different PKC isoforms.Pflugers Arch. 2024 Jul;476(7):1041-1064. doi: 10.1007/s00424-024-02966-5. Epub 2024 Apr 24. Pflugers Arch. 2024. PMID: 38658400
-
Not So Dry After All: DRY Mutants of the AT1A Receptor and H1 Receptor Can Induce G-Protein-Dependent Signaling.ACS Omega. 2020 Feb 3;5(6):2648-2659. doi: 10.1021/acsomega.9b03146. eCollection 2020 Feb 18. ACS Omega. 2020. PMID: 32095688 Free PMC article.
-
Optical approaches for single-cell and subcellular analysis of GPCR-G protein signaling.Anal Bioanal Chem. 2019 Jul;411(19):4481-4508. doi: 10.1007/s00216-019-01774-6. Epub 2019 Mar 30. Anal Bioanal Chem. 2019. PMID: 30927013 Free PMC article. Review.
-
TRUPATH, an open-source biosensor platform for interrogating the GPCR transducerome.Nat Chem Biol. 2020 Aug;16(8):841-849. doi: 10.1038/s41589-020-0535-8. Epub 2020 May 4. Nat Chem Biol. 2020. PMID: 32367019 Free PMC article.
References
-
- Vassilatis DK, Hohmann JG, Zeng H, Li F, Ranchalis JE, Mortrud MT, et al. The G protein-coupled receptor repertoires of human and mouse. Proc Natl Acad Sci U S A. 2003;100: 4903–8. doi: 10.1073/pnas.0230374100 - DOI - PMC - PubMed
-
- Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol Rev. 2005;85: 1159–204. doi: 10.1152/physrev.00003.2005 - DOI - PubMed
-
- Rens-Domiano S, Hamm HE. Structural and functional relationships of heterotrimeric G-proteins. FASEB J. 1995;9: 1059–66. Available: http://www.ncbi.nlm.nih.gov/pubmed/7649405 - PubMed
-
- Wedegaertner PB, Wilson PT, Bourne HR. Lipid modifications of trimeric G proteins. J Biol Chem. 1995;270: 503–6. Available: http://www.ncbi.nlm.nih.gov/pubmed/7822269 - PubMed
-
- Hepler JR, Gilman AG. G proteins. Trends Biochem Sci. 1992;17: 383–7. Available: http://www.ncbi.nlm.nih.gov/pubmed/1455506 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

