Higher versus lower amino acid intake in parenteral nutrition for newborn infants
- PMID: 29505664
- PMCID: PMC6494253
- DOI: 10.1002/14651858.CD005949.pub2
Higher versus lower amino acid intake in parenteral nutrition for newborn infants
Abstract
Background: Sick newborn and preterm infants frequently are not able to be fed enterally, necessitating parenteral fluid and nutrition. Potential benefits of higher parenteral amino acid (AA) intake for improved nitrogen balance, growth, and infant health may be outweighed by the infant's ability to utilise high intake of parenteral AA, especially in the days after birth.
Objectives: The primary objective is to determine whether higher versus lower intake of parenteral AA is associated with improved growth and disability-free survival in newborn infants receiving parenteral nutrition.Secondary objectives include determining whether:• higher versus lower starting or initial intake of amino acids is associated with improved growth and disability-free survival without side effects;• higher versus lower intake of amino acids at maximal intake is associated with improved growth and disability-free survival without side effects; and• increased amino acid intake should replace non-protein energy intake (glucose and lipid), should be added to non-protein energy intake, or should be provided simultaneously with non-protein energy intake.We conducted subgroup analyses to look for any differences in the effects of higher versus lower intake of amino acids according to gestational age, birth weight, age at commencement, and condition of the infant, or concomitant increases in fluid intake.
Search methods: We used the standard search strategy of the Cochrane Neonatal Review Group to search the Cochrane Central Register of Controlled Trials (2 June 2017), MEDLINE (1966 to 2 June 2017), Embase (1980 to 2 June 2017), and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1982 to 2 June 2017). We also searched clinical trials databases, conference proceedings, and citations of articles.
Selection criteria: Randomised controlled trials of higher versus lower intake of AAs as parenteral nutrition in newborn infants. Comparisons of higher intake at commencement, at maximal intake, and at both commencement and maximal intake were performed.
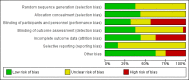
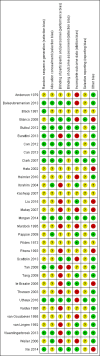
Data collection and analysis: Two review authors independently selected trials, assessed trial quality, and extracted data from included studies. We performed fixed-effect analyses and expressed treatment effects as mean difference (MD), risk ratio (RR), and risk difference (RD) with 95% confidence intervals (CIs) and assessed the quality of evidence using the GRADE approach.
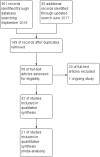
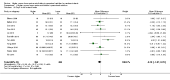
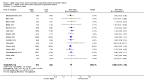
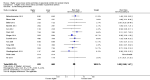
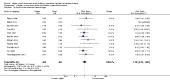
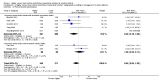
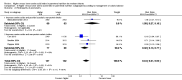
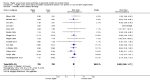
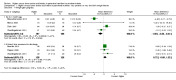
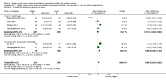
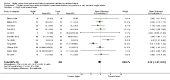
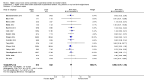
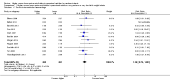
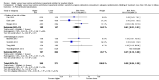
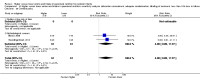
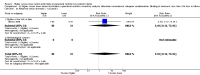
Main results: Thirty-two studies were eligible for inclusion. Six were short-term biochemical tolerance studies, one was in infants at > 35 weeks' gestation, one in term surgical newborns, and three yielding no usable data. The 21 remaining studies reported clinical outcomes in very preterm or low birth weight infants for inclusion in meta-analysis for this review.Higher AA intake had no effect on mortality before hospital discharge (typical RR 0.90, 95% CI 0.69 to 1.17; participants = 1407; studies = 14; I2 = 0%; quality of evidence: low). Evidence was insufficient to show an effect on neurodevelopment and suggest no reported benefit (quality of evidence: very low). Higher AA intake was associated with a reduction in postnatal growth failure (< 10th centile) at discharge (typical RR 0.74, 95% CI 0.56 to 0.97; participants = 203; studies = 3; I2 = 22%; typical RD -0.15, 95% CI -0.27 to -0.02; number needed to treat for an additional beneficial outcome (NNTB) 7, 95% CI 4 to 50; quality of evidence: very low). Subgroup analyses found reduced postnatal growth failure in infants that commenced on high amino acid intake (> 2 to ≤ 3 g/kg/day); that occurred with increased amino acid and non-protein caloric intake; that commenced on intake at < 24 hours' age; and that occurred with early lipid infusion.Higher AA intake was associated with a reduction in days needed to regain birth weight (MD -1.14, 95% CI -1.73 to -0.56; participants = 950; studies = 13; I2 = 77%). Data show varying effects on growth parameters and no consistent effects on anthropometric z-scores at any time point, as well as increased growth in head circumference at discharge (MD 0.09 cm/week, 95% CI 0.06 to 0.13; participants = 315; studies = 4; I2 = 90%; quality of evidence: very low).Higher AA intake was not associated with effects on days to full enteral feeds, late-onset sepsis, necrotising enterocolitis, chronic lung disease, any or severe intraventricular haemorrhage, or periventricular leukomalacia. Data show a reduction in retinopathy of prematurity (typical RR 0.44, 95% CI 0.21 to 0.93; participants = 269; studies = 4; I2 = 31%; quality of evidence: very low) but no difference in severe retinopathy of prematurity.Higher AA intake was associated with an increase in positive protein balance and nitrogen balance. Potential biochemical intolerances were reported, including risk of abnormal blood urea nitrogen (typical RR 2.77, 95% CI 2.13 to 3.61; participants = 688; studies = 7; I2 = 6%; typical RD 0.26, 95% CI 0.20 to 0.32; number needed to treat for an additional harmful outcome (NNTH) 4; 95% CI 3 to 5; quality of evidence: high). Higher amino acid intake in parenteral nutrition was associated with a reduction in hyperglycaemia (> 8.3 mmol/L) (typical RR 0.69, 95% CI 0.49 to 0.96; participants = 505; studies = 5; I2 = 68%), although the incidence of hyperglycaemia treated with insulin was not different.
Authors' conclusions: Low-quality evidence suggests that higher AA intake in parenteral nutrition does not affect mortality. Very low-quality evidence suggests that higher AA intake reduces the incidence of postnatal growth failure. Evidence was insufficient to show an effect on neurodevelopment. Very low-quality evidence suggests that higher AA intake reduces retinopathy of prematurity but not severe retinopathy of prematurity. Higher AA intake was associated with potentially adverse biochemical effects resulting from excess amino acid load, including azotaemia. Adequately powered trials in very preterm infants are required to determine the optimal intake of AA and effects of caloric balance in parenteral nutrition on the brain and on neurodevelopment.
Conflict of interest statement
None.
Figures
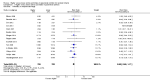


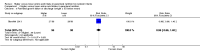

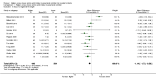




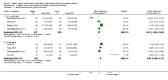











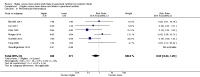

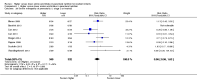





















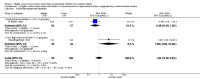


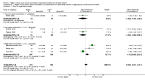




















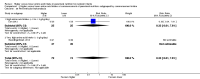
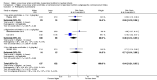
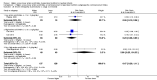

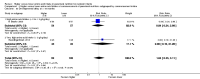



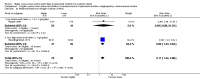

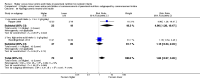







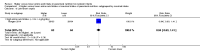








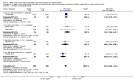









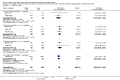




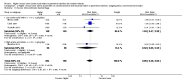
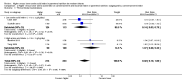







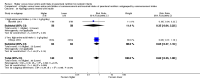


























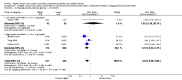


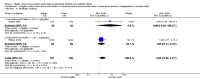


















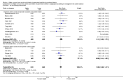

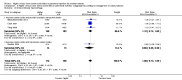


























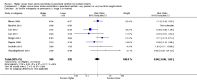

























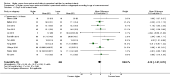

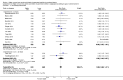



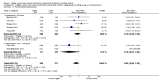

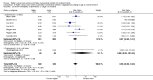































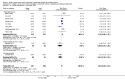



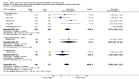
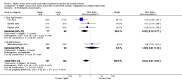
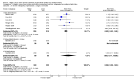








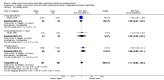


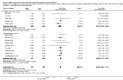


















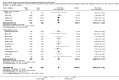















Comment in
-
Commentary on "Higher versus Lower Amino Acid Intake in Parenteral Nutrition for Newborn Infants".Neonatology. 2019;116(1):92-96. doi: 10.1159/000495913. Epub 2019 May 23. Neonatology. 2019. PMID: 31121599 No abstract available.
References
References to studies included in this review
Anderson 1979 {published data only}
-
- Anderson TL, Muttart CR, Bieber MA, Nicholson JF, Heird WC. A controlled trial of glucose versus glucose and amino acids in premature infants. Journal of Pediatrics 1979;94(6):947‐51. - PubMed
Balasubramanian 2013 {published data only}
-
- Balasubramanian H, Nanavati RN, Kabra NS. Effect of two different doses of parenteral amino acid supplementation on postnatal growth of very low birth weight neonates ‐ a randomized controlled trial. Indian Pediatrics 2013;50(12):1131‐6. - PubMed
Black 1981 {published data only}
-
- Black DD, Suttle EA, Whitington PF, Whitington GL, Korones SD. The effect of short‐term total parenteral nutrition on hepatic function in the human neonate: a prospective randomized study demonstrating alteration of hepatic canalicular function. Journal of Pediatrics 1981;99(3):445‐9. - PubMed
Blanco 2008 {published data only}
-
- Blanco CL, Falck A, Green BK, Cornell JE, Gong AK. Metabolic responses to early and high protein supplementation in a randomized trial evaluating the prevention of hyperkalemia in extremely low birth weight infants. Journal of Pediatrics 2008;153(4):535‐40. - PubMed
-
- Blanco CL, Gong AK, Green BK, Falck A, Schoolfield J, Liechty EA. Early changes in plasma amino acid concentrations during aggressive nutritional therapy in extremely low birth weight infants. Journal of Pediatrics 2011;158(4):543‐8 e1. - PubMed
-
- Blanco CL, Gong AK, Schoolfield J, Green BK, Daniels W, Liechty EA, et al. Impact of early and high amino acid supplementation on ELBW infants at 2 years. Journal of Pediatric Gastroenterology and Nutrition 2012;54(5):601‐7. - PubMed
Bulbul 2012 {published data only}
-
- Bulbul A, Okan F, Bulbul L, Nuhoglu A. Effect of low versus high early parenteral nutrition on plasma amino acid profiles in very low birth‐weight infants. Journal of Maternal‐Fetal & Neonatal Medicine 2012;25(6):770‐6. - PubMed
Burattini 2013 {published data only}
-
- Burattini I, Bellagamba MP, Spagnoli C, D'Ascenzo R, Mazzoni N, Peretti A, et al. Marche Neonatal Network. Targeting 2.5 versus 4 g/kg/day of amino acids for extremely low birth weight infants: a randomized clinical trial. Journal of Pediatrics 2013;163(5):1278‐82 e1. - PubMed
Can 2012 {published data only}
-
- Can E, Bulbul A, Uslu S, Comert S, Bolat F, Nuhoglu A. Effects of aggressive parenteral nutrition on growth and clinical outcome in preterm infants. Pediatrics International 2012;54:869‐74. - PubMed
Can 2013 {published data only}
-
- Can E, Bulbul A, Uslu S, Bolat F, Comert S, Nuhoglu A. Early aggressive parenteral nutrition induced high insulin‐like growth factor 1 (IGF‐1) and insulin‐like growth factor binding protein 3 (IGFBP3) levels can prevent risk of retinopathy of prematurity. Iranian Journal of Pediatrics 2013;23(4):403‐10. - PMC - PubMed
Clark 2007 {published data only}
-
- Clark RH, Chace DH, Spitzer AR, Pediatrix Amino Acid Study Group. Effects of two different doses of amino acid supplementation on growth and blood amino acid levels in premature neonates admitted to the neonatal intensive care unit: a randomized, controlled trial. Pediatrics 2007;120(6):1286‐96. - PubMed
-
- Kelleher AS, Clark RH, Steinbach M, Chace DH, Spitzer AR. The influence of amino‐acid supplementation, gestational age and time on thyroxine levels in premature neonates. Journal of Perinatology 2008;28(4):270‐4. - PubMed
Hata 2002 {published data only}
-
- Hata S, Kubota A, Okada A. A pediatric amino acid solution for total parenteral nutrition does not affect liver function test results in neonates. Surgery Today 2002;32:800‐3. - PubMed
Heimler 2010 {published data only}
-
- Heimler R, Bamberger JM, Sasidharan P. The effects of early parenteral amino acids on sick premature infants. Indian Journal of Pediatrics 2010;77:1395‐9. - PubMed
Ibrahim 2004 {published data only}
-
- Ibrahim HM, Jeroudi MA, Baier RJ, Dhanireddy R, Krouskop RW. Aggressive early total parental nutrition in low‐birth‐weight infants. Journal of Perinatology 2004;24:482‐6. - PubMed
Kashyap 2007 {published data only}
-
- Holuba AM, Bateman DA, Kashyap S. Effects of early higher protein intake on parenteral nutrition‐associated cholestasis in very low birth weight (BW <1250g) infants. Pediatric Research. 2011; Vol. PAS proceedings.
-
- Holuba AM, Genkinger JM, Kashyap S. Effects of early higher protein intake on retinopathy of prematurity in very low birth weight (BW <1250g) infants. Pediatric Research. 2013; Vol. PAS proceedings.
-
- Jensen EA, Bateman DA, Saiman L, Kashyap S. Effect of early aggressive nutrition on percutaneous central venous catheter (PCVL) associated blood stream Infections in infants with birth weight (BW) <1250g. Pediatric Research. 2010; Vol. PAS proceedings.
-
- Kashyap S. Is the early and aggressive administration of protein to very low birth weight infants safe and efficacious?. Current Opinion in Pediatrics 2008;20:132‐6. - PubMed
-
- Kashyap S, Abildskov K, Holleran SF. Effects of early aggressive nutrition in infants birth weight <1250 g: a randomized controlled trial. Pediatric Research. 2007; Vol. PAS proceedings.
Liu 2015 {published data only}
-
- Liu ZJ, Liu GS, Chen YG, Zhang HL, Wu XF. Value of early application of different doses of amino acids in parenteral nutrition among preterm infants. Zhongguo Dang Dai Er Ke za Zhi [Chinese Journal of Contemporary Pediatrics] 2015;17(1):53‐7. - PubMed
Makay 2007 {published data only}
-
- Makay B, Duman N, Ozer E, Kumral A, Yesilirmak D, Ozkan H. Randomized, controlled trial of early intravenous nutrition for prevention of neonatal jaundice in term and near‐term neonates. Journal of Pediatric Gastroenterology and Nutrition 2007;44:354‐8. - PubMed
Morgan 2014 {published data only}
-
- Burgess L, Flanagan B, Turner M, Morgan C. Elevated essential amino acid levels in very preterm infants receiving total parenteral nutrition. Journal of Pediatric Gastroenterology and Nutrition 2017;64:797.
-
- Mayes K, Tan M, Morgan C. Effect of hyperalimentation and insulin‐treated hyperglycemia on tyrosine levels in very preterm infants receiving parenteral nutrition. Journal of Parenteral and Enteral Nutrition 2014;38(1):92‐8. - PubMed
-
- Morgan C, Burgess L. High protein intake does not prevent low plasma levels of conditionally essential amino acids in very preterm infants receiving parenteral nutrition. Journal of Parenteral and Enteral Nutrition 2017;41:455‐62. - PubMed
-
- Morgan C, Herwitker S, Badhawi I, Hart A, Tan M, Mayes K, et al. SCAMP: standardised, concentrated, additional macronutrients, parenteral nutrition in very preterm infants: a phase IV randomised, controlled exploratory study of macronutrient intake, growth and other aspects of neonatal care. BMC Pediatrics 2011;11:53. - PMC - PubMed
-
- Morgan C, McGowan P, Herwitker S, Hart AE, Turner MA. Postnatal head growth in preterm infants: a randomized controlled parenteral nutrition study. Pediatrics 2014;133:e120‐8. - PubMed
Murdock 1995 {published data only}
-
- Forsyth JS, Murdock N, Crighton A. Low birthweight infants and total parenteral nutrition immediately after birth. III. Randomised study of energy substrate utilisation, nitrogen balance, and carbon dioxide production. Archives of Disease in Childhood. Fetal and Neonatal Edition 1995;73(1):F13‐6. - PMC - PubMed
-
- Murdock N, Crighton A, Nelson LM, Forsyth JS. Low birthweight infants and total parenteral nutrition immediately after birth. II. Randomised study of biochemical tolerance of intravenous glucose, amino acids, and lipid. Archives of Disease in Childhood. Fetal and Neonatal Edition 1995;73(1):F8‐12. - PMC - PubMed
Pappoe 2009 {published data only}
-
- Pappoe TA, Wu S‐Y, Pyati S. A randomized controlled trial comparing an aggressive and a conventional parenteral nutrition regimen in very low birth weight infants. Journal of Neonatal‐Perinatal Medicine 2009; Vol. 2, issue 3:149‐56. [CN‐00835899]
-
- Pappoe TA, Wu S‐Y, Pyati S. Rapid vs slow advancement of parenteral nutrition in preterm infants with birth weight less than 1250 g. Pediatric Academic Societies Conference Proceedings 2006:Abstract 5572.441. [CN‐00836071]
Pildes 1973 {published data only}
-
- Pildes RS, Ramamurthy RS, Cordero GV, Wong PW. Intravenous supplementation of L‐amino acids and dextrose in low‐birth‐weight infants. Journal of Pediatrics 1973;82(6):945‐50. - PubMed
Rivera 1993 {published data only}
-
- Rivera A, Bell EF, Bier DM. Effect of intravenous amino acids on protein metabolism of preterm infants during the first three days of life. Pediatric Research 1993;33(2):106‐11. - PubMed
Scattolin 2013 {published data only}
-
- Scattolin S, Gaio P, Betto M, Palatron S, Terlizzi F, Intini F, et al. Parenteral amino acid intakes: possible influences of higher intakes on growth and bone status in preterm infants. Journal of Perinatology 2013;33(1):33‐9. - PubMed
Tan 2008 {published data only}
-
- Burgess L, Morgan C, Mayes K, Tan M. Plasma arginine levels and blood glucose control in very preterm infants receiving 2 different parenteral nutrition regimens. Journal of Parenteral and Enteral Nutrition 2014;38(2):243‐53. - PubMed
-
- Tan M, Abernethy L, Cooke R. Improving head growth in preterm infants ‐ a randomised controlled trial II: MRI and developmental outcomes in the first year. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008;93(5):F342‐6. - PubMed
-
- Tan MJ, Cooke RW. Improving head growth in very preterm infants ‐ a randomised controlled trial I: neonatal outcomes. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008;93(5):F337‐41. - PubMed
Tang 2009 {published data only}
-
- Tang ZF, Huang Y, Zhang R, Chen C. Intensive early amino acid supplementation is efficacious and safe in the management of preterm infants. Zhonghua Er Ke za Zhi. Chinese Journal of Pediatrics 2009;47(3):209‐15. - PubMed
te Braake 2005 {published data only}
-
- Braake FW, Schierbeek H, Groof K, Vermes A, Longini M, Buonocore G, at al. Glutathione synthesis rates after amino acid administration directly after birth in preterm infants. American Journal of Clinical Nutrition 2008;88(2):333‐9. - PubMed
-
- Braake FW, Akker CH, Wattimena DJ, Huijmans JG, Goudoever JB. Amino acid administration to premature infants directly after birth. Journal of Pediatrics 2005;147(4):457‐61. - PubMed
-
- Akker CH, Braake FW, Schierbeek H, Rietveld T, Wattimena DJ, Bunt JE, et al. Albumin synthesis in premature neonates is stimulated by parenterally administered amino acids during the first days of life. American Journal of Clinical Nutrition 2007;86(4):1003‐8. - PubMed
-
- Akker CH, Braake FW, Wattimena DJ, Voortman G, Schierbeek H, Vermes A, et al. Effects of early amino acid administration on leucine and glucose kinetics in premature infants. Pediatric Research 2006;59(5):732‐5. - PubMed
-
- Akker CH, Braake FW, Weisglas‐Kuperus N, Goudoever JB. Observational outcome results following a randomized controlled trial of early amino acid administration in preterm infants. Journal of Pediatric Gastroenterology and Nutrition 2014;59(6):714‐9. - PubMed
Thureen 2003 {published data only}
-
- Thureen PJ, Melara D, Fennessey PV, Hay Jr WW. Effect of low versus high intravenous amino acid intake on very low birth weight infants in the early neonatal period. Pediatric Research 2003;53:24‐32. - PubMed
Uthaya 2016 {published data only}
-
- Uthaya S, Liu X, Babalis D, Dore CJ, Warwick J, Bell J, et al. Nutritional evaluation and optimisation in neonates: a randomized, double‐blind controlled trial of amino acid regimen and intravenous lipid composition in preterm parenteral nutrition. American Journal of Clinical Nutrition 2016;103:1443‐52. - PMC - PubMed
Vaidya 1995 {published data only}
-
- Vaidya UV, Bhave SA, Pandit AN. Parenteral nutrition (PN) in the management of very low birth weight (VLBW) babies ‐ a randomized controlled trial. Indian Pediatrics 1995;32(2):165‐70. - PubMed
van Goudoever 1995 {published data only}
-
- Goudoever JB, Colen T, Wattimena JL, Huijmans JG, Carnielli VP, Sauer PJ. Immediate commencement of amino acid supplementation in preterm infants: effect on serum amino acid concentrations and protein kinetics on the first day of life. Journal of Pediatrics 1995;127(3):458‐65. - PubMed
van Lingen 1992 {published data only}
-
- Lingen RA, Goudoever JB, Luijendijk IH, Wattimena JL, Sauer PJ. Effects of early amino acid administration during total parenteral nutrition on protein metabolism in pre‐term infants. Clinical Science 1992;82(2):199‐203. - PubMed
Vlaardingerbroek 2013 {published data only}
-
- Roelants JA, Vlaardingerbroek H, Akker CH, Jonge RC, Goudoever JB, Vermeulen MJ. Two‐year follow‐up of a randomized controlled nutrition intervention trial in very low‐birth‐weight infants. Journal of Parenteral and Enteral Nutrition 2016 Nov 1:148607116678196. - PubMed
-
- Vlaardingerbroek H, Roelants JA, Rook D, Dorst K, Schierbeek H, Vermes A, et al. Adaptive regulation of amino acid metabolism on early parenteral lipid and high‐dose amino acid administration in VLBW infants ‐ a randomized, controlled trial. Clinical Nutrition 2014;33(6):982‐90. - PubMed
-
- Vlaardingerbroek H, Schierbeek H, Rook D, Vermeulen MJ, Dorst K, Vermes A, et al. Albumin synthesis in very low birth weight infants is enhanced by early parenteral lipid and high‐dose amino acid administration. Clinical Nutrition 2016;35(2):344‐50. - PubMed
-
- Vlaardingerbroek H, Vermeulen MJ, Rook D, Akker CH, Dorst K, Wattimena JL, et al. Safety and efficacy of early parenteral lipid and high‐dose amino acid administration to very low birth weight infants. Journal of Pediatrics 2013;163(3):638‐44 e1‐5. - PubMed
Weiler 2006 {published data only}
-
- Weiler HA, Fitzpatrick‐Wong SC, Schellenberg JM, Fair DE, McCloy UR, Veitch RR, et al. Minimal enteral feeding within 3 d of birth in prematurely born infants with birth weight < or = 1200 g improves bone mass by term age. American Journal of Clinical Nutrition 2006;83(1):155‐62. - PubMed
Xie 2014 {published data only}
-
- Xie E, Sun J, Shen Y, Ju H, Li J, Zhang G, et al. Influence of early rapidly increased amino acid dosaging on nitrogen balance and growth in preterm infants. Chinese Journal of Clinical Nutrition 2014;22(3):136‐40.
References to studies excluded from this review
Abitbol 1975 {published data only}
-
- Abitbol CL, Feldman DB, Ahmann P, Rudman D. Plasma amino acid patterns during supplemental intravenous nutrition of low birth weight infants. Journal of Pediatrics 1975;86(5):766‐72. - PubMed
Adamkin 1991 {published data only}
-
- Adamkin DH, McClead RE Jr, Desai NS, McCulloch KM, Marchildon MB. Comparison of two neonatal intravenous amino acid formulations in preterm infants: a multicenter study. Journal of Perinatology 1991;11(4):375‐82. - PubMed
Adamkin 1995 {published data only}
-
- Adamkin DD, Radmacher P, Rosen P. Comparison of a neonatal versus general‐purpose amino acid formulation in preterm neonates. Journal of Perinatology 1995;15(2):108‐13. - PubMed
Alo 2010 {published data only}
-
- Alo D, Shahidullah M, Mannan MA, Noor K. Effect of parenteral amino acid supplementation in preterm low birth weight newborn. Mymensingh Medical Journal 2010;19(3):386‐90. - PubMed
Bellagamba 2016 {published data only}
-
- Bellagamba MP, Carmenati E, D'Ascenzo R, Malatesta M, Spagnoli C, Biagetti C, et al. One extra gram of protein to preterm infants from birth to 1800 g: a single‐blinded randomized clinical trial. Journal of Pediatric Gastroenterology and Nutrition 2016;62:879‐84. - PubMed
Brown 1989 {published data only}
-
- Brown MR, Thunberg BJ, Golub L, Maniscalco WM, Cox C, Shapiro DL. Decreased cholestasis with enteral instead of intravenous protein in the very low‐birth‐weight infant. Journal of Pediatric Gastroenterology and Nutrition 1989;9(1):21‐7. - PubMed
Bryan 1973 {published data only}
-
- Bryan MH, Wei P, Hamilton JR, Chance GW, Swyer PR. Supplemental intravenous alimentation in low‐birth‐weight infants. Journal of Pediatrics 1973;82(6):940‐4. - PubMed
Burger 1980 {published data only}
-
- Bürger U, Fritsch U, Bauer M, Peltner HU. Comparison of two amino acid mixtures for total parenteral nutrition of premature infants receiving assisted ventilation. Journal of Parenteral and Enteral Nutrition 1980;4(3):290‐3. - PubMed
Chessex 1985 {published data only}
-
- Chessex P, Zebiche H, Pineault M, Lepage D, Dallaire L. Effect of amino acid composition of parenteral solutions on nitrogen retention and metabolic response in very‐low‐birth weight infants. Journal of Pediatrics 1985;106(1):111‐7. - PubMed
Iacobelli 2010 {published data only}
-
- Iacobelli S, Bonsante F, Vintejoux A, Gouyon JB. Standardized parenteral nutrition in preterm infants: early impact on fluid and electrolyte balance. Neonatology 2010;98(1):84‐90. - PubMed
Kadrofske 2006 {published data only}
Loughead 1996 {unpublished data only}
-
- Loughead JL, Mezoff AG, Nevin‐Folino N. Parenteral nutrition in the VLBW newborn: comparison of two amino acid solutions. Pediatric Research. 1996; Vol. 39:315.
McIntosh 1990 {published data only}
-
- McIntosh N, Crockford H, Portnoy S, Berger M. Outcome at three years of sick neonates involved in a double‐blind trial of two parenteral amino acid preparations. Developmental Medicine and Child Neurology 1995;37:221‐5. - PubMed
Moltu 2014 {published data only}
-
- Blakstad EW, Strommen K, Moltu SJ, Wattam‐Bell J, Nordheim T, Almaas AN, et al. Improved visual perception in very low birth weight infants on enhanced nutrient supply. Neonatology 2015;108(1):30‐7. - PubMed
-
- Moltu SJ, Strommen K, Blakstad EW, Almaas AN, Westerberg AC, Braekke K, et al. Enhanced feeding in very‐low‐birth‐weight infants may cause electrolyte disturbances and septicemia ‐ a randomized, controlled trial. Clinical Nutrition 2013;32(2):207‐12. - PubMed
-
- Strommen K, Blakstad EW, Moltu SJ, Almaas AN, Westerberg AC, Amlien IK, et al. Enhanced nutrient supply to very low birth weight infants is associated with improved white matter maturation and head growth. Neonatology 2015;107(1):68‐75. - PubMed
Ogata 1983 {published data only}
-
- Ogata ES, Boehm JJ, Deddish RB, Wiringa KS, Yanagi RB, Bussey ME. Clinical trial of a 6.5% amino acid infusion in appropriate‐for‐gestational‐age premature neonates. Acta Chirurgica Scandinavica Supplementum 1983;517:39‐48. - PubMed
Parimi 2005 {published data only}
-
- Parimi PS, Kadrofske MM, Gruca LL, Hanson RW, Kalhan SC. Amino acids, glutamine, and protein metabolism in very low birth weight infants. Pediatric Research 2005;58(6):1259‐64. - PubMed
Rosenthal 1987 {published data only}
-
- Rosenthal M, Sinha S, Laywood E, Levene M. A double blind comparison of a new paediatric amino acid solution in neonatal total parenteral nutrition. Early Human Development 1987;15(3):137‐46. - PubMed
Rosenthal 1988 {published data only}
-
- Rosenthal M. Changes in urinary amino acid fractional excretion in neonates undergoing total parenteral nutrition. Early Human Development 1988;18(1):37‐44. - PubMed
Salle 1987 {published data only}
-
- Salle B, Rigo J, Senterre J. Parenteral nutrition in prematures. Adjustment of the amino‐acid intake. Archives Francaises de Pediatrie 1987;44(1):5‐8. - PubMed
Savich 1988 {published data only}
-
- Savich RD, Finley SL, Ogata ES. Intravenous lipid and amino acids briskly increase plasma glucose concentrations in small premature infants. American Journal of Perinatology 1988;5(3):201‐5. - PubMed
van Goudoever 1994 {published data only}
-
- Goudoever JB, Sulkers EJ, Timmerman M, Huijmans JG, Langer K, Carnielli VP, et al. Amino acid solutions for premature neonates during the first week of life: the role of N‐acetyl‐L‐cysteine and N‐acetyl‐L‐tyrosine. JPEN. Journal of Parenteral and Enteral Nutrition 1994;18(5):404‐8. - PubMed
Wilson 1997 {published data only}
Yeung 2003 {published data only}
-
- Yeung MY, Smyth JP, Maheshwari R, Shah S. Evaluation of standardized versus individualized total parenteral nutrition regime for neonates less than 33 weeks gestation. Journal of Paediatrics and Child Health 2003;39(8):613‐7. - PubMed
References to ongoing studies
Additional references
AAP 1998
-
- American Academy of Pediatrics, Committee on Nutrition. Paediatric Nutrition Handbook. 4th Edition. Elk Grove Village, IL: American Academy of Pediatrics, 1998.
AAP 2004
-
- American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004;114(1):297‐316. - PubMed
AAP 2009
-
- AAP Committee on Nutrition. In: Kleinman RE editor(s). Pediatric Nutrition Handbook. 6th Edition. Media, PA: AAP eBooks, 2009.
Andronikou 1983
-
- Andronikou S, Rothberg AD, Pettifor JM, Thomson PD. Early introduction of parenteral nutrition in premature infants and its effect on calcium and phosphate homeostasis. South African Medical Journal 1983;64(10):349‐51. - PubMed
Cauderay 1988
-
- Cauderay M, Schutz Y, Micheli JL, Calame A, Jéquier E. Energy‐nitrogen balances and protein turnover in small and appropriate for gestational age low birthweight infants. European Journal of Clinical Nutrition 1988;42(2):125‐36. - PubMed
Denne 1996
Duvanel 1999
-
- Duvanel CB, Fawer CL, Cotting J, Hohlfeld P, Matthieu JM. Long‐term effects of neonatal hypoglycemia on brain growth and psychomotor development in small‐for‐gestational‐age preterm infants. Journal of Pediatrics 1999;134(4):492‐8. - PubMed
Ehrenkranz 1999
-
- Ehrenkranz R, Younes N, Lemons J, Fanaroff AA, Donovan EF, Wright LL, et al. Longitudinal growth of hospitalized very low birthweight infants. Pediatrics 1999;104(2):280‐9. - PubMed
Ehrenkranz 2006
-
- Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 2006;117(4):1253‐61. - PubMed
Embleton 2001
-
- Embleton NE, Pang N, Cooke RJ. Postnatal malnutrition and growth retardation: an inevitable consequence of current recommendations in preterm infants?. Pediatrics 2001;107(2):270‐3. - PubMed
EPSGHAN 2005
-
- Koletzko B, Goulet O, Hunt J, Krohn K, Shamir R, Parenteral Nutrition Guidelines Working Group, European Society for Clinical Nutrition and Metabolism, European Society of Paediatric Gastroenterology, Hepatology, Nutrition (ESPGHAN). European Society of Paediatric Research (ESPR). Guidelines on Paediatric Parenteral Nutrition of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the European Society for Clinical Nutrition and Metabolism (ESPEN), supported by the European Society of Paediatric Research (ESPR). Journal of Pediatric Gastroenterology and Nutrition 2005;41 Suppl 2:S1‐87. - PubMed
Fomon 1993
-
- Fomon SJ. Nutrition of Normal Infants. St Louis, MO: Mosby, 1993.
Fusch 2009
GRADEpro 2008 [Computer program]
-
- Brozek J, Oxman A, Schünemann H. GRADEproGDT. Version 3.2 for Windows. The GRADE Working Group, 2008.
Gresham 1971
-
- Gresham EL, Simons PS, Battaglia FC. Maternal‐fetal urea concentration difference in man: metabolic significance. Journal of Pediatrics 1971;79(5):809‐11. - PubMed
Guyatt 2011a
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] - PubMed
Guyatt 2011b
-
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence ‐ study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21247734] - PubMed
Guyatt 2011c
-
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] - PubMed
Guyatt 2011d
-
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence ‐ inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] - PubMed
Guyatt 2011e
-
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence ‐ indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] - PubMed
Hay 1996
-
- Hay WW. Assessing the effect of disease on nutrition of the preterm infant. Clinical Biochemistry 1996;5(5):399‐417. - PubMed
Hays 2006
-
- Hays SP, Smith EO, Sunehag AL. Hyperglycemia is a risk factor for early death and morbidity in extremely low birth‐weight infants. Pediatrics 2006;118:1811‐8. - PubMed
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hozo 2005
Inder 2003
-
- Inder TE, Wells SJ, Mogridge NB, Spencer C, Volpe JJ. Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. Journal of Pediatrics 2003;143(2):171‐9. - PubMed
International Committee 2005
-
- International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Archives of Ophthalmology 2005;123(7):991‐9. - PubMed
Jakobsson 1990
-
- Jakobsson B, Aperia A. High protein intake accelerates the growth of Na,K ATPase in rat renal tubules. Acta Physiologica Scandinavica 1990;139(1):1‐7. - PubMed
Kashyap 1994
-
- Kashyap S, Schulze KF, Ramakrishna R, Dell RB, Heird WC. Evaluation of a mathematical model for predicting the relationship between protein and energy intakes of low‐birth‐weight infants and the rate and composition of weight gain. Pediatric Research 1994;35(6):704‐12. - PubMed
Kashyap 1994a
-
- Kashyap S, Heird WC. Protein requirements of low birthweight, very low birthweight and small for gestational age infants. In: Raiha NCR editor(s). Protein Metabolism During Infancy. New York: Raven Press, 1994:133‐51.
Koch 1968
-
- Koch G, Wendel H. Adjustment of arterial blood gases and acid base balance in the normal newborn infant during the first week of life. Biology of the Neonate 1968;12:136‐61. - PubMed
Lemons 1976
Lemons 2001
-
- Lemons JA, Bauer CR, Oh W, Korones SB, Papile LA, Stoll BJ, et al. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, January 1995 through December 1996. NICHD Neonatal Research Network. Pediatrics 2001;107(1):E1. - PubMed
Lucas 1988
Lucas 1994
Lucas 1998
Micheli 1993
-
- Micheli JL, Schutz Y. Nutritional Needs of the Preterm Infant. Scientific Basis and Practical Guidelines. Pawling, NY: Caduceus Medical Publishers, Inc., 1993:29‐46.
Murray 1993
-
- Murray BM, Campos SP, Schoenl M, MacGillvray MH. Effect of dietary protein intake on renal growth; possible role of insulin‐like growth factor‐I. Journal of Laboratory and Clinical Medicine 1993;122(6):677‐85. - PubMed
Nayak 1989
-
- Nayak KC, Sethi AS, Aggarwal TD, Chadda VS, Kumar KK. Bactericidal power of neutrophils in protein calorie malnutrition. Indian Journal of Paediatrics 1989;56(3):371‐7. - PubMed
Ong 2007
-
- Ong KK. Catch‐up growth in small for gestational age babies: good or bad?. Current Opinion in Endocrinology, Diabetes and Obesity 2007;14(1):30‐4. - PubMed
Papile 1978
-
- Papile L, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular haemorrhage: a study of infants with birth weights less than 1500 grams. Journal of Pediatrics 1978;92(4):529‐34. - PubMed
Raiha 2001
-
- Raiha NC, Fazzolari A, Cayozzo C, Puccio G, Minoli I, Moro G, et al. Protein nutrition during infancy: effects on growth and metabolism. Nutrition and Growth. Baltimore, MD: Lippincott William and Wilkins, 2001:73‐83.
Reading 1990
-
- Reading RF, Ellis R, Fleetwood A. Plasma albumin and total protein in preterm babies from birth to eight weeks. Early Human Development 1990;22(2):81‐7. - PubMed
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ridout 2005
-
- Ridout E, Melara D, Rottinghaus S, Thureen PJ. Blood urea nitrogen concentration as a marker of amino‐acid intolerance in neonates with birthweight less than 1250 g. Journal of Perinatology 2005;25(2):130‐3. - PubMed
Rolland 1995
-
- Rolland‐Cachera MF, Deheegen M, Akrout M, Bellisle F. Influence of macronutrients on adiposity and development ‐ a follow up study of nutrition and growth from ten months to eight years of age. International Journal of Obesity and Related Metabolic Disorders 1995;19(8):573‐8. - PubMed
Scaglioni 2000
-
- Scaglioni S, Agostoni C, Notaris RD, Radaelli G, Radice N, Valenti M, et al. Early macronutrient intake and overweight at five years of age. International Journal of Obesity and Related Metabolic Disorders 2000;24(6):777‐81. - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GWG. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. Updated October 2013. Available from www.guidelinedevelopment.org/handbook.
Senterre 1983
-
- Senterre J, Vover M, Putet C, Rigo J. Nitrogen, fat and mineral balance studies in preterm infants fed banked human milk, a human milk formula or a low‐birthweight infant formula. Nestle Nutrition Workshop Series. New York: Raven Press, 1983:102‐11.
Simmer 2006
te Braake 2007
-
- Braake FW, Akker CH, Riedijk MA, Goudoever JB. Parenteral amino acid and energy administration to premature infants in early life. Seminars in Fetal & Neonatal Medicine 2007;12(1):11‐8. - PubMed
Thureen 2003
-
- Thureen PJ, Melara D, Fennessey PV, Hay WW Jr. Effect of low versus high intravenous amino acid intake on very low birthweight infants in the early neonatal period. Pediatric Research 2003;53(1):24‐32. - PubMed
Thureen 1999
-
- Thureen PJ. Early aggressive nutrition in the neonate. Pediatrics in Review 1999;20(9):e45‐55. - PubMed
Trivedi 2013
-
- Trivedi A, Sinn JK. Early versus late administration of amino acids in preterm infants receiving parenteral nutrition. Cochrane Database of Systematic Reviews 2013;7:CD008771. [doi: 10.1002/14651858.CD008771.pub2] - PubMed
Usmani 1993
-
- Usmani SS, Cavaliere T, Casatelli J, Harper RG. Plasma ammonia levels in very low birth weight preterm infants. Journal of Pediatrics 1993;123(5):797‐800. - PubMed
Ziegler 1991
-
- Ziegler E. Malnutrition in the premature infant. Acta Paediatrica Scandinavica. Supplement 1991;374:58‐66. - PubMed
Ziegler 1994
-
- Ziegler EE. Protein in premature feeding. Nutrition 1994;10(1):69‐71. - PubMed
Zlotkin 1987
-
- Zlotkin SH, Casselman CW. Percentile estimates of reference values for total protein and albumin in sera of premature infants (<37 weeks of gestation). Clinical Chemistry 1987;33(3):411‐3. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

