Neuronal Transforming Growth Factor beta Signaling via SMAD3 Contributes to Pain in Animal Models of Chronic Pancreatitis
- PMID: 29505748
- PMCID: PMC5985212
- DOI: 10.1053/j.gastro.2018.02.030
Neuronal Transforming Growth Factor beta Signaling via SMAD3 Contributes to Pain in Animal Models of Chronic Pancreatitis
Abstract
Background & aims: Chronic pancreatitis (CP) is characterized by pancreatic inflammation and fibrosis, associated with increased pancreatic expression of transforming growth factor beta (TGFB). It is not clear how these might contribute to pain. We investigated whether TGFB signaling via SMAD induces sensitization of pancreatic sensory neurons to increase nociception.
Methods: CP was induced in Sprague-Dawley rats by infusion of trinitrobenzene sulfonic acid; some rats were given intrathecal infusions of TGFB1. CP was induced in control mice by administration of cerulein; we also studied β1glo/Ptf1acre-ER mice, which on induction overexpress TGFB1 in pancreatic acinar cells, and TGFBr1f/f-CGRPcreER mice, which have inducible disruption of TGFBr1 in calcitonin gene-related peptide-positive neurons. Dominant negative forms of human TGFBR2 and SMAD3 were overexpressed from viral vectors in rat pancreas. Some rats were given the SMAD3 inhibitors SIS3 or halofuginone. After induction of CP, mice were analyzed for pain in behavior tests or electrophysiologic studies of sensory neurons. Pancreatic nociceptor excitability was examined by patch-clamp techniques and nociception was measured by Von Frey Filament tests for referred somatic hyperalgesia and behavioral responses to pancreatic electrical stimulation. Pancreata were collected from mice and rats and analyzed histologically and by enzyme-linked immunosorbent assay and immunohistochemistry.
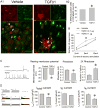
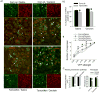
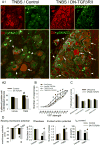
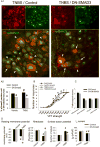
Results: Overexpression of TGFB in pancreatic acinar cells of mice and infusion of TGFB1 into rats resulted in sensory neuron hyperexcitability, SMAD3 activation, and increased nociception. This was accompanied by a reduction in the transient A-type current in pancreas-specific sensory neurons in rats, a characteristic of nociceptive sensitization in animal models of CP. Conversely, pancreata from TGFBr1f/f-CGRPcreER mice, rats with pancreatic expression of dominant negative forms of human TGFBR2 or SMAD3, and rats given small molecule inhibitors of SMAD3 had attenuated neuronal sensitization and pain behavior following induction of CP. In contrast to findings from peripheral administration of TGFB1, intrathecal infusion of TGFB1 reduced hyperalgesia in rats with CP.
Conclusions: In pancreata of mice and rats, TGFB promotes peripheral nociceptive sensitization via a direct effect on primary sensory neurons mediated by intra-neuronal SMAD3. This is distinct from the central nervous system, where TGFB reduces nociception. These results provide an explanation for the link between fibrosis and pain in patients with CP. This signaling pathway might be targeted therapeutically to reduce pain in patients with CP.
Keywords: Mouse Model; Neurobiology; Pain Signal Transduction; Pancreas.
Copyright © 2018 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures



Similar articles
-
Nucleus tractus solitarius mediates hyperalgesia induced by chronic pancreatitis in rats.World J Gastroenterol. 2019 Oct 28;25(40):6077-6093. doi: 10.3748/wjg.v25.i40.6077. World J Gastroenterol. 2019. PMID: 31686764 Free PMC article.
-
Amelioration of pancreatic fibrosis in mice with defective TGF-beta signaling.Pancreas. 2005 Apr;30(3):e71-9. doi: 10.1097/01.mpa.0000157388.54016.0a. Pancreas. 2005. PMID: 15782092
-
Nerve growth factor modulates TRPV1 expression and function and mediates pain in chronic pancreatitis.Gastroenterology. 2011 Jul;141(1):370-7. doi: 10.1053/j.gastro.2011.03.046. Epub 2011 Apr 5. Gastroenterology. 2011. PMID: 21473865 Free PMC article.
-
Pain mechanisms in chronic pancreatitis: of a master and his fire.Langenbecks Arch Surg. 2011 Feb;396(2):151-60. doi: 10.1007/s00423-010-0731-1. Epub 2010 Dec 10. Langenbecks Arch Surg. 2011. PMID: 21153480 Free PMC article. Review.
-
No major contribution of the TGFBR1- and TGFBR2-mediated pathway to Kabuki syndrome.Am J Med Genet A. 2006 Apr 15;140(8):903-5. doi: 10.1002/ajmg.a.31168. Am J Med Genet A. 2006. PMID: 16528739 Review. No abstract available.
Cited by
-
Role of the CXCR4/ALK5/Smad3 Signaling Pathway in Cancer-Induced Bone Pain.J Pain Res. 2020 Oct 14;13:2567-2576. doi: 10.2147/JPR.S260508. eCollection 2020. J Pain Res. 2020. PMID: 33116799 Free PMC article.
-
Cardiopulmonary and Neurologic Dysfunctions in Fibrodysplasia Ossificans Progressiva.Biomedicines. 2021 Feb 5;9(2):155. doi: 10.3390/biomedicines9020155. Biomedicines. 2021. PMID: 33562570 Free PMC article. Review.
-
Krüppel-like Factor 5 Plays an Important Role in the Pathogenesis of Chronic Pancreatitis.Cancers (Basel). 2023 Nov 15;15(22):5427. doi: 10.3390/cancers15225427. Cancers (Basel). 2023. PMID: 38001687 Free PMC article.
-
Gene Therapy for Pancreatic Diseases: Current Status.Int J Mol Sci. 2018 Oct 31;19(11):3415. doi: 10.3390/ijms19113415. Int J Mol Sci. 2018. PMID: 30384450 Free PMC article. Review.
-
TGF-β2 Induces Gli1 in a Smad3-Dependent Manner Against Cerebral Ischemia/Reperfusion Injury After Isoflurane Post-conditioning in Rats.Front Neurosci. 2019 Jun 26;13:636. doi: 10.3389/fnins.2019.00636. eCollection 2019. Front Neurosci. 2019. PMID: 31297044 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

