The in vitro effects of macrophages on the osteogenic capabilities of MC3T3-E1 cells encapsulated in a biomimetic poly(ethylene glycol) hydrogel
- PMID: 29505890
- PMCID: PMC7649064
- DOI: 10.1016/j.actbio.2018.02.026
The in vitro effects of macrophages on the osteogenic capabilities of MC3T3-E1 cells encapsulated in a biomimetic poly(ethylene glycol) hydrogel
Abstract
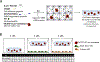
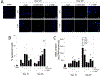
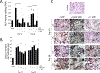
Poly(ethylene glycol) PEG-based hydrogels are promising for cell encapsulation and tissue engineering, but are known to elicit a foreign body response (FBR) in vivo. The goal of this study was to investigate the impact of the FBR, and specifically the presence of inflammatory macrophages, on encapsulated cells and their ability to synthesize new extracellular matrix. This study employed an in vitro co-culture system with murine macrophages and MC3T3-E1 pre-osteoblasts encapsulated in a bone-mimetic hydrogel, which were cultured in transwell inserts, and exposed to an inflammatory stimulant, lipopolysaccharide (LPS). The co-culture was compared to mono-cultures of the cell-laden hydrogels alone and with LPS over 28 days. Two macrophage cell sources, RAW 264.7 and primary derived, were investigated. The presence of LPS-stimulated primary macrophages led to significant changes in the cell-laden hydrogel by a 5.3-fold increase in percent apoptotic osteoblasts at day 28, 4.2-fold decrease in alkaline phosphatase activity at day 10, and 7-fold decrease in collagen deposition. The presence of LPS-stimulated RAW macrophages led to significant changes in the cell-laden hydrogel by 5-fold decrease in alkaline phosphatase activity at day 10 and 4-fold decrease in collagen deposition. Mineralization, as measured by von Kossa stain or quantified by calcium content, was not sensitive to macrophages or LPS. Elevated interleukin-6 and tumor necrosis factor-α secretion were detected in mono-cultures with LPS and co-cultures. Overall, primary macrophages had a more severe inhibitory effect on osteoblast differentiation than the macrophage cell line, with greater apoptosis and collagen I reduction. In summary, this study highlights the detrimental effects of macrophages on encapsulated cells for bone tissue engineering.
Statement of significance: Poly(ethylene glycol) (PEG)-based hydrogels are promising for cell encapsulation and tissue engineering, but are known to elicit a foreign body response (FBR) in vivo. The impact of the FBR on encapsulated cells and their ability to synthesize tissue has not been well studied. This study utilizes thiol-ene click chemistry to create a biomimetic, enzymatically degradable hydrogel system with which to encapsulate MC3T3-E1 pre-osteoblasts. The osteogenic capabilities and differentiation of these cellswerestudied in co-culture with macrophages, known drivers of the FBR.This study demonstrates that macrophages reduce osteogenic capabilities of encapsulated cellsin vitroand suggestthat the FBR should be considered for in vivo tissue engineering.
Keywords: Co-culture; Host response; MC3T3-E1 pre-osteoblast; Macrophage; Osteogenesis; Poly(ethylene glycol) hydrogel.
Copyright © 2018. Published by Elsevier Ltd.
Figures




Similar articles
-
The effects of hydroxyapatite nanoparticles embedded in a MMP-sensitive photoclickable PEG hydrogel on encapsulated MC3T3-E1 pre-osteoblasts.Biomed Mater. 2018 May 2;13(4):045009. doi: 10.1088/1748-605X/aabb31. Biomed Mater. 2018. PMID: 29611815 Free PMC article.
-
Inflammation via myeloid differentiation primary response gene 88 signaling mediates the fibrotic response to implantable synthetic poly(ethylene glycol) hydrogels.Acta Biomater. 2019 Dec;100:105-117. doi: 10.1016/j.actbio.2019.09.043. Epub 2019 Sep 27. Acta Biomater. 2019. PMID: 31568879 Free PMC article.
-
Immunomodulation by mesenchymal stem cells combats the foreign body response to cell-laden synthetic hydrogels.Biomaterials. 2015 Feb;41:79-88. doi: 10.1016/j.biomaterials.2014.11.020. Epub 2014 Dec 5. Biomaterials. 2015. PMID: 25522967 Free PMC article.
-
Beyond Encapsulation: Exploring Macrophage-Fibroblast Cross Talk in Implant-Induced Fibrosis.Tissue Eng Part B Rev. 2024 Dec;30(6):596-606. doi: 10.1089/ten.TEB.2023.0300. Epub 2024 Mar 27. Tissue Eng Part B Rev. 2024. PMID: 38420650 Review.
-
Mechanical properties of cellularly responsive hydrogels and their experimental determination.Adv Mater. 2010 Aug 17;22(31):3484-94. doi: 10.1002/adma.200904179. Adv Mater. 2010. PMID: 20473984 Free PMC article. Review.
Cited by
-
Piezo1-mediated M2 macrophage mechanotransduction enhances bone formation through secretion and activation of transforming growth factor-β1.Cell Prolif. 2023 Sep;56(9):e13440. doi: 10.1111/cpr.13440. Epub 2023 Mar 7. Cell Prolif. 2023. PMID: 36880296 Free PMC article.
-
Mechanosensitive Piezo1 in Periodontal Ligament Cells Promotes Alveolar Bone Remodeling During Orthodontic Tooth Movement.Front Physiol. 2021 Nov 22;12:767136. doi: 10.3389/fphys.2021.767136. eCollection 2021. Front Physiol. 2021. PMID: 34880779 Free PMC article.
-
Cell- and Serum-Derived Proteins Act as DAMPs to Activate RAW 264.7 Macrophage-like Cells on Silicone Implants.ACS Biomater Sci Eng. 2024 Mar 11;10(3):1418-1434. doi: 10.1021/acsbiomaterials.3c01393. Epub 2024 Feb 6. ACS Biomater Sci Eng. 2024. PMID: 38319825 Free PMC article.
-
Hydrogel-Based Scaffolds: Advancing Bone Regeneration Through Tissue Engineering.Gels. 2025 Feb 27;11(3):175. doi: 10.3390/gels11030175. Gels. 2025. PMID: 40136878 Free PMC article. Review.
-
Comparative Study of Two Common In Vitro Models for the Pancreatic Islet with MIN6.Tissue Eng Regen Med. 2023 Feb;20(1):127-141. doi: 10.1007/s13770-022-00507-8. Epub 2023 Jan 2. Tissue Eng Regen Med. 2023. PMID: 36592326 Free PMC article.
References
-
- Amer LD, Holtzinger A, Keller G, Mahoney MJ, Bryant SJ, Enzymatically degradable poly(ethylene glycol) hydrogels for the 3D culture and release of human embryonic stem cell derived pancreatic precursor cell aggregates, Acta Biomater. 22 (2015) 103–110. doi:10.1016/j.actbio.2015.04.013. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

