Influence of Sleep Stage on LH Pulse Initiation in the Normal Late Follicular Phase and in Polycystic Ovary Syndrome
- PMID: 29506013
- PMCID: PMC7053660
- DOI: 10.1159/000488110
Influence of Sleep Stage on LH Pulse Initiation in the Normal Late Follicular Phase and in Polycystic Ovary Syndrome
Abstract
Objective: During the early follicular phase, sleep-related luteinizing hormone (LH) pulse initiation is positively associated with brief awakenings but negatively associated with rapid eye movement (REM) sleep. The relationship between sleep architecture and LH pulse initiation has not been assessed in other cycle stages or in women with polycystic ovary syndrome (PCOS).
Design and methods: We performed concomitant frequent blood sampling (LH pulse analysis) and polysomnography on 8 normal women (cycle day 7-11) and 7 women with PCOS (at least cycle day 7).
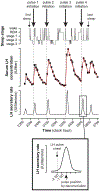
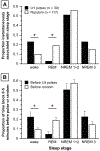
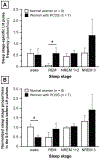
Results: In the normal women, the 5 min preceding LH pulses contained more wake epochs and fewer REM epochs than the 5 min preceding randomly determined time points (wake: 22.3 vs. 9.1%, p = 0.0111; REM: 4.4 vs. 18.8%, p = 0.0162). However, LH pulse initiation was not related to wake or REM epochs in PCOS; instead, the 5 min preceding LH pulses contained more slow-wave sleep (SWS) than the 5 min before random time points (20.9 vs. 6.7%, p = 0.0089). Compared to the normal subjects, the women with PCOS exhibited a higher REM-associated LH pulse frequency (p = 0.0443) and a lower proportion of wake epochs 0-5 min before LH pulses (p = 0.0205).
Conclusions: Sleep-related inhibition of LH pulse generation during the later follicular phase is normally weakened by brief awakenings and strengthened by REM sleep. In women with PCOS, LH pulse initiation is not appropriately discouraged by REM sleep and may be encouraged by SWS; these abnormalities may contribute to a high sleep-related LH pulse frequency in PCOS.
Keywords: Gonadotropin-releasing hormone; Luteinizing hormone; Polycystic ovary syndrome; Sleep.
© 2018 S. Karger AG, Basel.
Figures





Similar articles
-
Polycystic ovarian syndrome: evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone.J Clin Endocrinol Metab. 2000 Nov;85(11):4047-52. doi: 10.1210/jcem.85.11.6992. J Clin Endocrinol Metab. 2000. PMID: 11095431 Clinical Trial.
-
Polycystic ovary syndrome: evidence for reduced sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone.J Clin Endocrinol Metab. 1998 Feb;83(2):582-90. doi: 10.1210/jcem.83.2.4604. J Clin Endocrinol Metab. 1998. PMID: 9467578
-
Brief wake episodes modulate sleep-inhibited luteinizing hormone secretion in the early follicular phase.J Clin Endocrinol Metab. 2005 Apr;90(4):2050-5. doi: 10.1210/jc.2004-2033. Epub 2005 Jan 25. J Clin Endocrinol Metab. 2005. PMID: 15671093
-
Regulation of gonadotropin secretion: implications for polycystic ovary syndrome.Semin Reprod Med. 2002 Nov;20(4):317-26. doi: 10.1055/s-2002-36706. Semin Reprod Med. 2002. PMID: 12536355 Review.
-
The origins and sequelae of abnormal neuroendocrine function in polycystic ovary syndrome.Hum Reprod Update. 2006 Jul-Aug;12(4):351-61. doi: 10.1093/humupd/dml017. Epub 2006 May 2. Hum Reprod Update. 2006. PMID: 16670102 Review.
Cited by
-
Sleep and Puberty.Curr Opin Endocr Metab Res. 2021 Apr;17:1-7. doi: 10.1016/j.coemr.2020.09.009. Epub 2020 Oct 9. Curr Opin Endocr Metab Res. 2021. PMID: 35005296 Free PMC article.
-
Resistance to the Insulin and Elevated Level of Androgen: A Major Cause of Polycystic Ovary Syndrome.Front Endocrinol (Lausanne). 2021 Oct 20;12:741764. doi: 10.3389/fendo.2021.741764. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34745009 Free PMC article. Review.
-
Abnormal GnRH Pulsatility in Polycystic Ovary Syndrome: Recent Insights.Curr Opin Endocr Metab Res. 2020 Jun;12:78-84. doi: 10.1016/j.coemr.2020.04.005. Epub 2020 Apr 23. Curr Opin Endocr Metab Res. 2020. PMID: 32676541 Free PMC article.
-
The Relationship Between Progesterone, Sleep, and LH and FSH Secretory Dynamics in Early Postmenarchal Girls.J Clin Endocrinol Metab. 2019 Jun 1;104(6):2184-2194. doi: 10.1210/jc.2018-02400. J Clin Endocrinol Metab. 2019. PMID: 30649404 Free PMC article.
-
Association between Nocturnal Frequency and Erectile Function in Eugonadal Men with Benign Prostatic Obstruction: A Cross Sectional Study.World J Mens Health. 2021 Apr;39(2):338-345. doi: 10.5534/wjmh.190146. Epub 2020 Jan 29. World J Mens Health. 2021. PMID: 32202080 Free PMC article.
References
-
- Soules MR, Steiner RA, Cohen NL, Bremner WJ, Clifton DK. Nocturnal slowing of pulsatile luteinizing hormone secretion in women during the follicular phase of the menstrual cycle. J Clin Endocrinol Metab 1985; 61: 43–49. - PubMed
-
- Filicori M, Santoro N, Merriam GR, Crowley WF Jr. Characterization of the physiological pattern of episodic gonadotropin secretion throughout the human menstrual cycle. J Clin Endocrinol Metab 1986; 62: 1136–1144. - PubMed
-
- Rossmanith WG, Yen SS. Sleep-associated decrease in luteinizing hormone pulse frequency during the early follicular phase of the menstrual cycle: evidence for an opioidergic mechanism. J Clin Endocrinol Metab 1987; 65: 715–718. - PubMed
-
- Rossmanith WG, Liu CH, Laughlin GA, Mortola JF, Suh BY, Yen SS. Relative changes in LH pulsatility during the menstrual cycle: using data from hypogonadal women as a reference point. Clin Endocrinol (Oxf) 1990; 32: 647–660. - PubMed
-
- Rossmanith WG, Lauritzen C. The luteinizing hormone pulsatile secretion: diurnal excursions in normally cycling and postmenopausal women. Gynecol Endocrinol 1991; 5: 249–265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

