Cognition and dementia in older patients with epilepsy
- PMID: 29506031
- PMCID: PMC5972564
- DOI: 10.1093/brain/awy022
Cognition and dementia in older patients with epilepsy
Abstract
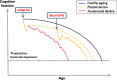
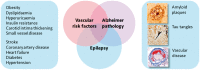
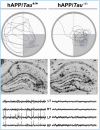
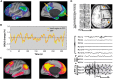
With advances in healthcare and an ageing population, the number of older adults with epilepsy is set to rise substantially across the world. In developed countries the highest incidence of epilepsy is already in people over 65 and, as life expectancy increases, individuals who developed epilepsy at a young age are also living longer. Recent findings show that older persons with epilepsy are more likely to suffer from cognitive dysfunction and that there might be an important bidirectional relationship between epilepsy and dementia. Thus some people with epilepsy may be at a higher risk of developing dementia, while individuals with some forms of dementia, particularly Alzheimer's disease and vascular dementia, are at significantly higher risk of developing epilepsy. Consistent with this emerging view, epidemiological findings reveal that people with epilepsy and individuals with Alzheimer's disease share common risk factors. Recent studies in Alzheimer's disease and late-onset epilepsy also suggest common pathological links mediated by underlying vascular changes and/or tau pathology. Meanwhile electrophysiological and neuroimaging investigations in epilepsy, Alzheimer's disease, and vascular dementia have focused interest on network level dysfunction, which might be important in mediating cognitive dysfunction across all three of these conditions. In this review we consider whether seizures promote dementia, whether dementia causes seizures, or if common underlying pathophysiological mechanisms cause both. We examine the evidence that cognitive impairment is associated with epilepsy in older people (aged over 65) and the prognosis for patients with epilepsy developing dementia, with a specific emphasis on common mechanisms that might underlie the cognitive deficits observed in epilepsy and Alzheimer's disease. Our analyses suggest that there is considerable intersection between epilepsy, Alzheimer's disease and cerebrovascular disease raising the possibility that better understanding of shared mechanisms in these conditions might help to ameliorate not just seizures, but also epileptogenesis and cognitive dysfunction.
Figures

Comment in
-
Reply: Late onset epilepsy and Alzheimer's disease: exploring the dual pathogenic role of amyloid-β.Brain. 2018 Aug 1;141(8):e61. doi: 10.1093/brain/awy163. Brain. 2018. PMID: 29893783 No abstract available.
-
Late onset epilepsy and Alzheimer's disease: exploring the dual pathogenic role of amyloid-β.Brain. 2018 Aug 1;141(8):e60. doi: 10.1093/brain/awy162. Brain. 2018. PMID: 29893897 No abstract available.
Similar articles
-
Dementia -- Caring, Ethics, Ethnical and Economical Aspects: A Systematic Review [Internet].Stockholm: Swedish Council on Health Technology Assessment (SBU); 2008 Jun. SBU Assessment No. 172. Stockholm: Swedish Council on Health Technology Assessment (SBU); 2008 Jun. SBU Assessment No. 172. PMID: 28876770 Free Books & Documents. Review.
-
Morphometric network differences in ageing versus Alzheimer's disease dementia.Brain. 2020 Feb 1;143(2):635-649. doi: 10.1093/brain/awz414. Brain. 2020. PMID: 32040564 Free PMC article.
-
Epilepsy, antiepileptic drugs and dementia.Curr Opin Neurol. 2020 Apr;33(2):191-197. doi: 10.1097/WCO.0000000000000802. Curr Opin Neurol. 2020. PMID: 32073437 Review.
-
Informing etiological heterogeneity of mild cognitive impairment and risk for progression to dementia with plasma p-tau217.J Prev Alzheimers Dis. 2025 Jan;12(1):100011. doi: 10.1016/j.tjpad.2024.100011. Epub 2025 Jan 1. J Prev Alzheimers Dis. 2025. PMID: 39800468 Free PMC article.
-
Profiles of neuropsychological impairment in autopsy-defined Alzheimer's disease and cerebrovascular disease.Brain. 2007 Mar;130(Pt 3):731-9. doi: 10.1093/brain/awl385. Epub 2007 Jan 31. Brain. 2007. PMID: 17267522
Cited by
-
β-hydroxybutyrate and its metabolic effects on age-associated pathology.Exp Mol Med. 2020 Apr;52(4):548-555. doi: 10.1038/s12276-020-0415-z. Epub 2020 Apr 8. Exp Mol Med. 2020. PMID: 32269287 Free PMC article. Review.
-
Late-Onset Epilepsy With Unknown Etiology: A Pilot Study on Neuropsychological Profile, Cerebrospinal Fluid Biomarkers, and Quantitative EEG Characteristics.Front Neurol. 2020 Apr 15;11:199. doi: 10.3389/fneur.2020.00199. eCollection 2020. Front Neurol. 2020. PMID: 32351438 Free PMC article.
-
Upsetting the Balance: How Modifiable Risk Factors Contribute to the Progression of Alzheimer's Disease.Biomolecules. 2024 Feb 24;14(3):274. doi: 10.3390/biom14030274. Biomolecules. 2024. PMID: 38540695 Free PMC article. Review.
-
Exploring biomarkers of neurodegeneration in epilepsy: Critical insights.Epileptic Disord. 2025 Jun;27(3):341-357. doi: 10.1002/epd2.70023. Epub 2025 Apr 8. Epileptic Disord. 2025. PMID: 40197800 Free PMC article. Review.
-
Vitexin Alleviates Kainic Acid-Induced Seizure Through Inhibiting P2X7R/NLRP3 Signaling Pathway.Inflammation. 2025 Jun 30. doi: 10.1007/s10753-025-02324-2. Online ahead of print. Inflammation. 2025. PMID: 40586851
References
-
- Amatniek JC, Hauser WA, DelCastillo-Castaneda C, Jacobs DM, Marder K, Bell K, et al.Incidence and predictors of seizures in patients with Alzheimer’s disease. Epilepsia 2006; 47: 867–72. - PubMed
-
- Annegers J, Hauser W, Coan SP, Rocca W. A population-based study of seizures after traumatic brain injuries. N Engl J Med 1998; 338: 20–4. - PubMed
-
- Annegers JF, Dubinsky S, Coan SP, Newmark ME, Roht L. The incidence of epilepsy and unprovoked seizures in multiethnic, urban health maintenance organizations. Epilepsia 1999; 40: 502–6. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

