Exocytosis Protein DOC2B as a Biomarker of Type 1 Diabetes
- PMID: 29506054
- PMCID: PMC6276681
- DOI: 10.1210/jc.2017-02492
Exocytosis Protein DOC2B as a Biomarker of Type 1 Diabetes
Abstract
Context: Efforts to preserve β-cell mass in the preclinical stages of type 1 diabetes (T1D) are limited by few blood-derived biomarkers of β-cell destruction.
Objective: Platelets are proposed sources of blood-derived biomarkers for a variety of diseases, and they show distinct proteomic changes in T1D. Thus, we investigated changes in the exocytosis protein, double C2 domain protein-β (DOC2B) in platelets and islets from T1D humans, and prediabetic nonobese diabetic (NOD) mice.
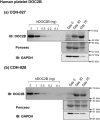
Design, patients, and main outcome measure: Protein levels of DOC2B were assessed in platelets and islets from prediabetic NOD mice and humans, with and without T1D. Seventeen new-onset T1D human subjects (10.3 ± 3.8 years) were recruited immediately following diagnosis, and platelet DOC2B levels were compared with 14 matched nondiabetic subjects (11.4 ± 2.9 years). Furthermore, DOC2B levels were assessed in T1D human pancreatic tissue samples, cytokine-stimulated human islets ex vivo, and platelets from T1D subjects before and after islet transplantation.
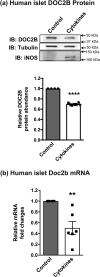
Results: DOC2B protein abundance was substantially reduced in prediabetic NOD mouse platelets, and these changes were mirrored in the pancreatic islets from the same mice. Likewise, human DOC2B levels were reduced over twofold in platelets from new-onset T1D human subjects, and this reduction was mirrored in T1D human islets. Cytokine stimulation of normal islets reduced DOC2B expression ex vivo. Remarkably, platelet DOC2B levels increased after islet transplantation in patients with T1D.
Conclusions: Reduction of DOC2B is an early feature of T1D, and DOC2B abundance may serve as a valuable in vivo indicator of β-cell mass and an early biomarker of T1D.
Figures


Similar articles
-
Pancreatic β-cells package double C2-like domain beta protein into extracellular vesicles via tandem C2 domains.Front Endocrinol (Lausanne). 2024 Oct 21;15:1451279. doi: 10.3389/fendo.2024.1451279. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39497805 Free PMC article.
-
DOC2b enrichment mitigates proinflammatory cytokine-induced CXCL10 expression by attenuating IKKβ and STAT-1 signaling in human islets.Metabolism. 2025 Mar;164:156132. doi: 10.1016/j.metabol.2025.156132. Epub 2025 Jan 11. Metabolism. 2025. PMID: 39805534
-
Pancreatic Alpha-Cells Contribute Together With Beta-Cells to CXCL10 Expression in Type 1 Diabetes.Front Endocrinol (Lausanne). 2020 Sep 15;11:630. doi: 10.3389/fendo.2020.00630. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33042009 Free PMC article.
-
Monitoring of beta cell replacement outcomes.Panminerva Med. 2016 Mar;58(1):59-71. Epub 2016 Jan 13. Panminerva Med. 2016. PMID: 26763742 Review.
-
Type 1 diabetes in mice and men: gene expression profiling to investigate disease pathogenesis.Immunol Res. 2014 May;58(2-3):340-50. doi: 10.1007/s12026-014-8501-8. Immunol Res. 2014. PMID: 24682832 Review.
Cited by
-
TC2N inhibits distant metastasis and stemness of breast cancer via blocking fatty acid synthesis.J Transl Med. 2024 Jan 2;22(1):6. doi: 10.1186/s12967-023-04721-3. J Transl Med. 2024. PMID: 38167440 Free PMC article.
-
DOC2b Enhances β-Cell Function via a Novel Tyrosine Phosphorylation-Dependent Mechanism.Diabetes. 2022 Jun 1;71(6):1246-1260. doi: 10.2337/db21-0681. Diabetes. 2022. PMID: 35377441 Free PMC article.
-
Conventional and Unconventional Mechanisms by which Exocytosis Proteins Oversee β-cell Function and Protection.Int J Mol Sci. 2021 Feb 12;22(4):1833. doi: 10.3390/ijms22041833. Int J Mol Sci. 2021. PMID: 33673206 Free PMC article. Review.
-
Setting the Stage for Insulin Granule Dysfunction during Type-1-Diabetes: Is ER Stress the Culprit?Biomedicines. 2022 Oct 25;10(11):2695. doi: 10.3390/biomedicines10112695. Biomedicines. 2022. PMID: 36359215 Free PMC article. Review.
-
Protecting functional β cells with a therapeutic peptide.Ann Transl Med. 2018 Sep;6(18):372. doi: 10.21037/atm.2018.07.26. Ann Transl Med. 2018. PMID: 30370299 Free PMC article. No abstract available.
References
-
- Siljander HT, Hermann R, Hekkala A, Lähde J, Tanner L, Keskinen P, Ilonen J, Simell O, Veijola R, Knip M. Insulin secretion and sensitivity in the prediction of type 1 diabetes in children with advanced β-cell autoimmunity. Eur J Endocrinol. 2013;169(4):479–485. - PubMed
-
- Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, Schandene L, Crenier L, De Block C, Seigneurin JM, De Pauw P, Pierard D, Weets I, Rebello P, Bird P, Berrie E, Frewin M, Waldmann H, Bach JF, Pipeleers D, Chatenoud L. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352(25):2598–2608. - PubMed
-
- Herold KC, Gitelman SE, Willi SM, Gottlieb PA, Waldron-Lynch F, Devine L, Sherr J, Rosenthal SM, Adi S, Jalaludin MY, Michels AW, Dziura J, Bluestone JA. Teplizumab treatment may improve C-peptide responses in participants with type 1 diabetes after the new-onset period: a randomised controlled trial. Diabetologia. 2013;56(2):391–400. - PMC - PubMed
-
- Gaisano HY. Recent new insights into the role of SNARE and associated proteins in insulin granule exocytosis. Diabetes Obes Metab. 2017;19(Suppl 1):115–123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

