The association between soluble klotho and cardiovascular parameters in chronic kidney disease: results from the KNOW-CKD study
- PMID: 29506503
- PMCID: PMC5838864
- DOI: 10.1186/s12882-018-0851-3
The association between soluble klotho and cardiovascular parameters in chronic kidney disease: results from the KNOW-CKD study
Abstract
Background: Klotho, a protein linked to aging, has emerged as a pivotal player in mineral bone metabolism and might explain the relationship between chronic kidney disease (CKD) and cardiovascular disease (CVD). The present study aimed to investigate the association between serum klotho and cardiac parameters from a large-scale Korean CKD cohort.
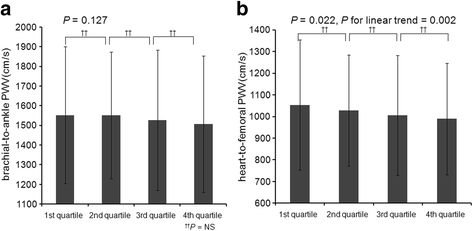
Methods: We analyzed 2101 participants from KoreaN Cohort Study for Outcome in Patients With Chronic Kidney Disease (KNOW-CKD) cohort who had been measured for serum klotho levels. Left ventricular hypertrophy evaluated by left ventricular mass index (LVMI) and arterial stiffness measured by brachial-to-ankle pulse wave velocity (baPWV) were explored as cardiovascular parameters.
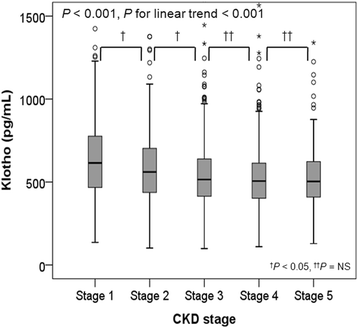
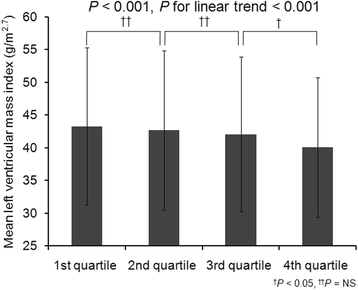
Results: Patients were 53.6 ± 12.2 years old and 61.1% were male. The mean estimated glomerular filtration rate (eGFR) was 53.0 ± 30.7 mL/min/1.73m2. The median serum klotho level was 536 (interquartile range [IQR]: 420-667) pg/mL. Advanced CKD stages were associated with lower serum klotho levels (P < 0.001, P for linear trend < 0.001). Ascending quartiles of klotho were significantly associated with decreased LMVI (P < 0.001, P for linear trend< 0.001). A multivariable linear regression model showed serum klotho had a significant inverse association with LVMI (β - 0.04; 95% CI [confidence interval] -0.004, - 0.00007; P = 0.041). However, there was no significant association between serum klotho and baPWV after adjustment (β 0.003; 95% CI -0.04, 0.05; P = 0.876).
Trial registration: This trial was registered on ClinicalTrials.gov on 28 June 2012 ( NCT01630486 ).
Conclusions: Serum klotho was an independent biomarker of LVMI, but not arterial stiffness.
Keywords: Chronic kidney disease; Left ventricular hypertrophy; Left ventricular mass index; Pulse wave velocity; Serum klotho; Soluble klotho.
Conflict of interest statement
Ethics approval and consent to participate
The study protocol was approved by the ethical committee of each participating clinical center, including the Institutional Review Boards of Seoul National University Hospital, Severance Hospital, Kangbuk Samsung Medical Center, Seoul St. Mary’s Hospital, Gil Hospital, Eulji General Hospital, Chonnam National University Hospital, and Busan Paik Hospital. All participating patients provided written informed consent. The study protocol was in accordance with the principles of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Middleton RJ, Parfrey PS, Foley RN. Left ventricular hypertrophy in the renal patient. J Am Soc Nephrol. 2001;12:1079–1084. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

