A phase IIb randomized, chronic-dosing, incomplete block, cross-over study of glycopyrronium, delivered via metered dose inhaler, compared with a placebo and an active control in patients with moderate-to-severe COPD
- PMID: 29506504
- PMCID: PMC5838878
- DOI: 10.1186/s12931-018-0739-6
A phase IIb randomized, chronic-dosing, incomplete block, cross-over study of glycopyrronium, delivered via metered dose inhaler, compared with a placebo and an active control in patients with moderate-to-severe COPD
Abstract
Background: Long-acting muscarinic antagonist (LAMA) and long-acting β2-agonist (LABA) bronchodilators are key to the pharmacologic treatment of chronic obstructive pulmonary disease (COPD). This Phase IIb study investigated the safety and efficacy of four doses of the LAMA glycopyrronium (GP) delivered using co-suspension delivery technology via metered dose inhaler (MDI). The study was part of a wider clinical trial program performed to determine the optimal dose of GP MDI, the LABA formoterol fumarate dihydrate (FF) MDI, and glycopyrronium/formoterol fumarate dihydrate (GFF) MDI fixed-dose combination to be taken forward into Phase III studies.
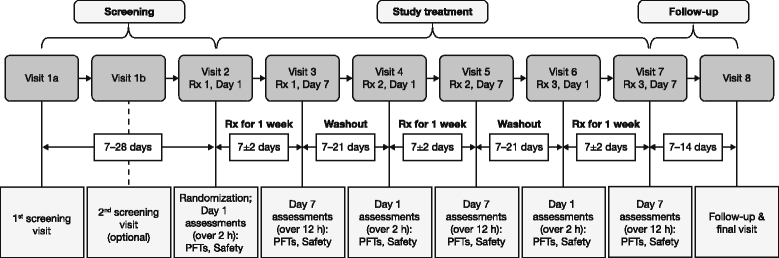
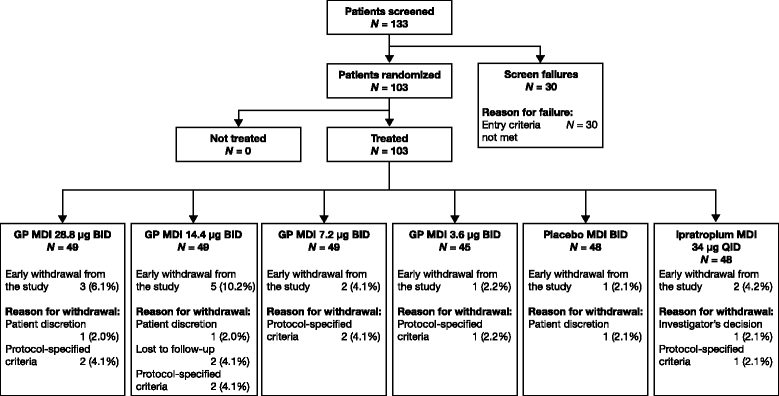
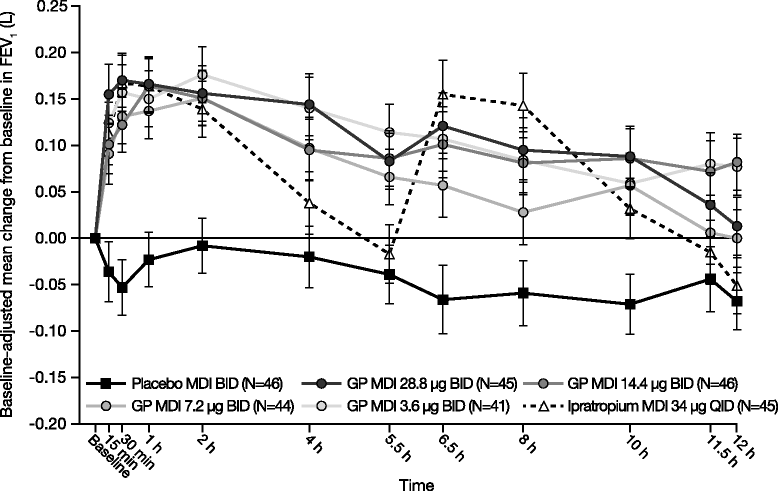
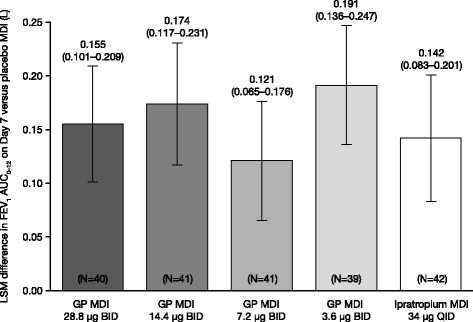
Methods: In this randomized, double-blind, 7-day chronic-dosing, three-period incomplete block, cross-over study, patients with moderate-to-severe COPD received two of the four doses of GP MDI (28.8 μg, 14.4 μg, 7.2 μg, and 3.6 μg) twice daily (BID), and either placebo MDI BID or open-label ipratropium MDI 34 μg four times daily. The primary efficacy endpoint was forced expiratory volume in 1 s (FEV1) area under the curve from 0 to 12 h (AUC0-12) relative to baseline on Day 7. Secondary and exploratory efficacy endpoints were assessed on Days 1 and 7. Safety and tolerability were evaluated throughout the study.
Results: All GP MDI treatments were superior to placebo MDI for the primary efficacy endpoint (all p < 0.0001). However, only GP MDI 28.8 μg and 14.4 μg demonstrated statistical superiority to placebo MDI for all secondary efficacy endpoints analyzed in this study, with the exception of GP MDI 14.4 μg versus placebo MDI for the proportion of patients achieving ≥12% improvement in FEV1. No nominally significant differences were observed between GP MDI 28.8 μg and GP MDI 14.4 μg for any of the endpoints. All doses of GP MDI were well tolerated, with no unexpected safety findings.
Conclusions: This study indicated that there was no advantage of GP MDI 28.8 μg compared with GP MDI 14.4 μg. It therefore added to the evidence from the Phase I/II clinical trial program, which identified GP MDI 14.4 μg as the most appropriate dose for use in the Phase III clinical studies.
Trial registration: ClinicalTrials.gov (NCT01350128). Registered May 09, 2011.
Keywords: Bronchodilator; Chronic obstructive pulmonary disease; Co-suspension delivery technology; Glycopyrronium; Long-acting muscarinic antagonist; Long-acting β2-agonist; Metered dose inhaler.
Conflict of interest statement
Ethics approval and consent to participate
This study was conducted in accordance with Good Clinical Practice Guidelines including the International Conference on Harmonisation, the US Code of Federal Regulations, and the Declaration of Helsinki. An institutional review board (Independent Investigational Review Board, Inc., FL, USA; IRB00003563) approved the protocol and informed consent form, and written informed consent was obtained from patients prior to screening.
Consent for publication
Not applicable.
Competing interests
EMK has served on advisory boards, speaker panels, or received travel reimbursement from Amphastar, AstraZeneca, Forest, Mylan, Novartis, Oriel, Sunovion, Teva, and Theravance. He has conducted multicenter clinical trials for ~ 40 pharmaceutical companies.
SS serves on the speaker’s bureau for Boehringer Ingelheim, Mylan, Pearl – A member of the AstraZeneca Group, and Sunovion.
CK has no potential conflicts of interest to disclose.
ESR is an employee of Pearl – A member of the AstraZeneca Group.
CR is Chief Executive Officer of Pearl – A member of the AstraZeneca Group, and an employee of AstraZeneca.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
A randomized, seven-day study to assess the efficacy and safety of a glycopyrrolate/formoterol fumarate fixed-dose combination metered dose inhaler using novel Co-Suspension™ Delivery Technology in patients with moderate-to-very severe chronic obstructive pulmonary disease.Respir Res. 2017 Jan 6;18(1):8. doi: 10.1186/s12931-016-0491-8. Respir Res. 2017. PMID: 28061907 Free PMC article. Clinical Trial.
-
Efficacy and safety of four doses of glycopyrrolate/formoterol fumarate delivered via a metered dose inhaler compared with the monocomponents in patients with moderate-to-severe COPD.Int J Chron Obstruct Pulmon Dis. 2018 Jun 19;13:1965-1977. doi: 10.2147/COPD.S166455. eCollection 2018. Int J Chron Obstruct Pulmon Dis. 2018. PMID: 29950826 Free PMC article. Clinical Trial.
-
Pharmacokinetics of glycopyrronium/formoterol fumarate dihydrate delivered via metered dose inhaler using co-suspension delivery technology in patients with moderate-to-very severe COPD.Int J Chron Obstruct Pulmon Dis. 2018 Mar 21;13:945-953. doi: 10.2147/COPD.S154988. eCollection 2018. Int J Chron Obstruct Pulmon Dis. 2018. PMID: 29606861 Free PMC article. Clinical Trial.
-
Systematic review and network meta-analysis of the efficacy and safety of glycopyrrolate/formoterol fumarate metered dose inhaler in comparison with other long-acting muscarinic antagonist/long-acting β2-agonist fixed-dose combinations in COPD.Ther Adv Respir Dis. 2019 Jan-Dec;13:1753466619894502. doi: 10.1177/1753466619894502. Ther Adv Respir Dis. 2019. PMID: 31868101 Free PMC article.
-
Budesonide/Glycopyrronium/Formoterol: A Review in COPD.Drugs. 2021 Aug;81(12):1411-1422. doi: 10.1007/s40265-021-01562-6. Epub 2021 Aug 3. Drugs. 2021. PMID: 34342835 Free PMC article. Review.
Cited by
-
Glycopyrronium/Formoterol: A Review in COPD.Drugs. 2019 Sep;79(13):1455-1466. doi: 10.1007/s40265-019-01186-x. Drugs. 2019. PMID: 31468315 Review.
-
Inhaled glycopyrrolate for the treatment of chronic obstructive pulmonary disease.Int J Chron Obstruct Pulmon Dis. 2018 Jun 12;13:1873-1888. doi: 10.2147/COPD.S162646. eCollection 2018. Int J Chron Obstruct Pulmon Dis. 2018. PMID: 29928118 Free PMC article. Review.
References
-
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of COPD. 2018. http://www.goldcopd.org. Accessed 23 Jan 2018.
-
- Doty A, Schroeder J, Vang K, Sommerville M, Taylor M, Flynn B, et al. Drug delivery from an innovative LAMA/LABA co-suspension delivery technology fixed-dose combination MDI: evidence of consistency, robustness, and reliability. AAPS PharmSciTech. 2018;19:837–844. doi: 10.1208/s12249-017-0891-1. - DOI - PubMed
-
- AstraZeneca Pharmaceuticals LP . Bevespi aerosphere™ prescribing information. 2017.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

