RNAseq analysis of bronchial epithelial cells to identify COPD-associated genes and SNPs
- PMID: 29506519
- PMCID: PMC5838965
- DOI: 10.1186/s12890-018-0603-y
RNAseq analysis of bronchial epithelial cells to identify COPD-associated genes and SNPs
Abstract
Background: There is a need for more powerful methods to identify low-effect SNPs that contribute to hereditary COPD pathogenesis. We hypothesized that SNPs contributing to COPD risk through cis-regulatory effects are enriched in genes comprised by bronchial epithelial cell (BEC) expression patterns associated with COPD.
Methods: To test this hypothesis, normal BEC specimens were obtained by bronchoscopy from 60 subjects: 30 subjects with COPD defined by spirometry (FEV1/FVC < 0.7, FEV1% < 80%), and 30 non-COPD controls. Targeted next generation sequencing was used to measure total and allele-specific expression of 35 genes in genome maintenance (GM) genes pathways linked to COPD pathogenesis, including seven TP53 and CEBP transcription factor family members. Shrinkage linear discriminant analysis (SLDA) was used to identify COPD-classification models. COPD GWAS were queried for putative cis-regulatory SNPs in the targeted genes.
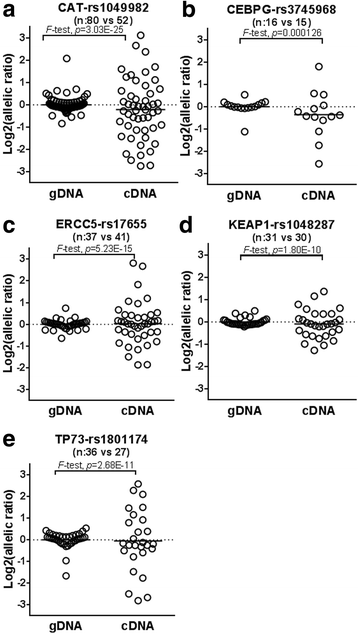
Results: On a network basis, TP53 and CEBP transcription factor pathway gene pair network connections, including key DNA repair gene ERCC5, were significantly different in COPD subjects (e.g., Wilcoxon rank sum test for closeness, p-value = 5.0E-11). ERCC5 SNP rs4150275 association with chronic bronchitis was identified in a set of Lung Health Study (LHS) COPD GWAS SNPs restricted to those in putative regulatory regions within the targeted genes, and this association was validated in the COPDgene non-hispanic white (NHW) GWAS. ERCC5 SNP rs4150275 is linked (D' = 1) to ERCC5 SNP rs17655 which displayed differential allelic expression (DAE) in BEC and is an expression quantitative trait locus (eQTL) in lung tissue (p = 3.2E-7). SNPs in linkage (D' = 1) with rs17655 were predicted to alter miRNA binding (rs873601). A classifier model that comprised gene features CAT, CEBPG, GPX1, KEAP1, TP73, and XPA had pooled 10-fold cross-validation receiver operator characteristic area under the curve of 75.4% (95% CI: 66.3%-89.3%). The prevalence of DAE was higher than expected (p = 0.0023) in the classifier genes.
Conclusions: GM genes comprised by COPD-associated BEC expression patterns were enriched for SNPs with cis-regulatory function, including a putative cis-rSNP in ERCC5 that was associated with COPD risk. These findings support additional total and allele-specific expression analysis of gene pathways with high prior likelihood for involvement in COPD pathogenesis.
Keywords: Bronchial epithelial cells; CAT; CEBPG; COPD; ERCC5; GPX1; GWAS; KEAP1; TP73; XPA; cis-regulation; eQTL.
Conflict of interest statement
Ethics approval and consent to participate
Collection and use of samples and corresponding medical/demographic data was approved under University of Toledo IRB protocols #108538 and #107844. Each subject included in this study provided written informed consent.
Consent for publication
Not applicable.
Competing interests
JCW is a consultant for and has equity interest in Accugenomics, Inc. which has a financial interest in the data presented here. JCW, TB and ELC have issued and pending patents for the technology and biomarkers presented here. Other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
-
- Cho MH, McDonald ML, Zhou X, Mattheisen M, Castaldi PJ, Hersh CP, Demeo DL, Sylvia JS, Ziniti J, Laird NM, et al. Risk loci for chronic obstructive pulmonary disease: a genome-wide association study and meta-analysis. Lancet Respir Med. 2014;2(3):214–225. doi: 10.1016/S2213-2600(14)70002-5. - DOI - PMC - PubMed
-
- Qiao D, Lange C, Beaty TH, Crapo JD, Barnes KC, Bamshad M, Hersh CP, Morrow J, Pinto-Plata VM, Marchetti N, et al. Exome sequencing analysis in severe, early-onset chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2016;193(12):1353–1363. doi: 10.1164/rccm.201506-1223OC. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

