Tearing down the walls: FDA approves next generation sequencing (NGS) assays for actionable cancer genomic aberrations
- PMID: 29506529
- PMCID: PMC5838869
- DOI: 10.1186/s13046-018-0702-x
Tearing down the walls: FDA approves next generation sequencing (NGS) assays for actionable cancer genomic aberrations
Abstract
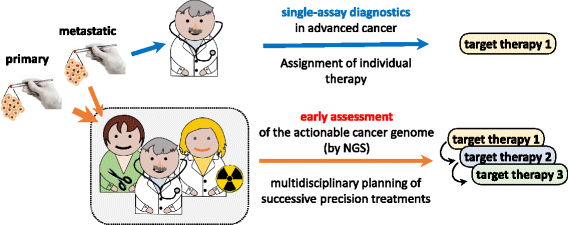
The United States Food and Drug Administration (FDA) recently approved the clinical use of two comprehensive 'mid-size' Next Generation Sequencing (NGS) panels calling actionable genomic aberrations in cancer. This is the first endorsement, by a regulatory body, of a new standard of care in oncology. Herein, we argue that besides its many practice-changing implications, this approval tears down the conceptual walls dividing system biology from clinical practice, diagnosis from research, prevention from therapy, cancer genetics from cancer genomics, and computational biology from empirical therapy assignment.
Keywords: Bioinformatics; Cancer screening and prevention; Ethics; Genomic aberrations; Next generation sequencing (NGS); Patient advocacy; Precision medicine; Regulatory issues.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Hyman DM, Solit DB, Arcila ME, Cheng DT, Sabbatini P, Baselga J, et al. Precision medicine at memorial Sloan Kettering cancer center: clinical next-generation sequencing enabling next-generation targeted therapy trials. Drug Discov Today. 2015;20(12):1422–1428. doi: 10.1016/j.drudis.2015.08.005. - DOI - PMC - PubMed
-
- Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, et al. Memorial Sloan Kettering-integrated mutation profiling of actionable cancer targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17(3):251–264. doi: 10.1016/j.jmoldx.2014.12.006. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

