Experimental malaria-associated acute respiratory distress syndrome is dependent on the parasite-host combination and coincides with normocyte invasion
- PMID: 29506544
- PMCID: PMC5839036
- DOI: 10.1186/s12936-018-2251-3
Experimental malaria-associated acute respiratory distress syndrome is dependent on the parasite-host combination and coincides with normocyte invasion
Abstract
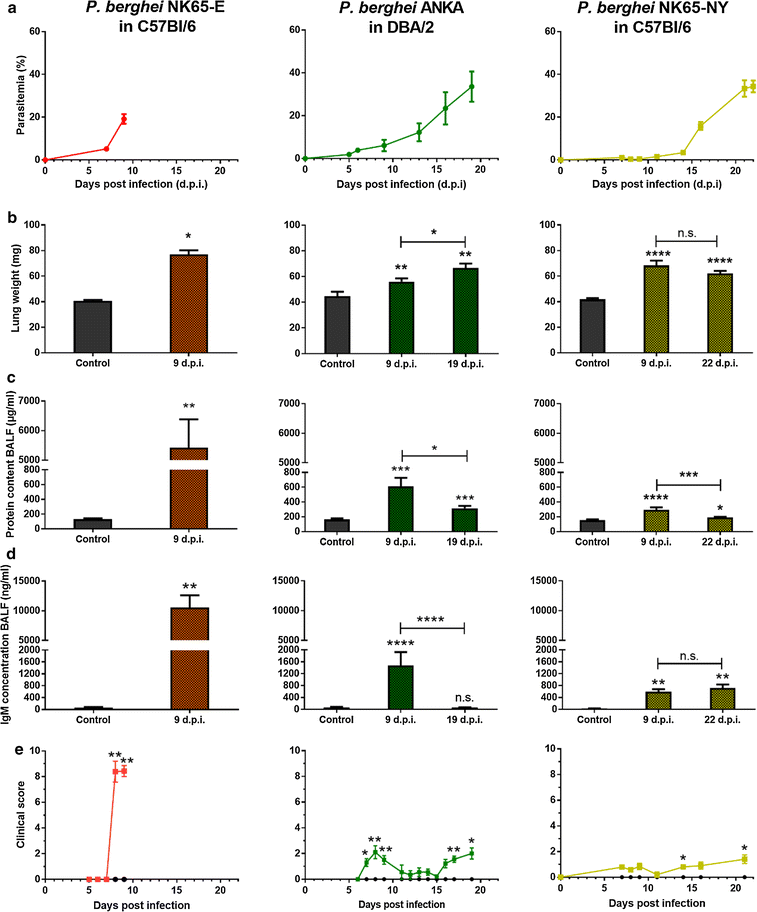
Background: Malaria-associated acute respiratory distress syndrome (MA-ARDS) is a complication of malaria with a lethality rate of up to 80% despite anti-malarial treatment. It is characterized by a vast infiltration of leukocytes, microhaemorrhages and vasogenic oedema in the lungs. Previously, a mouse model for MA-ARDS was developed by infection of C57BL/6 mice with the Edinburgh line NK65-E of Plasmodium berghei.
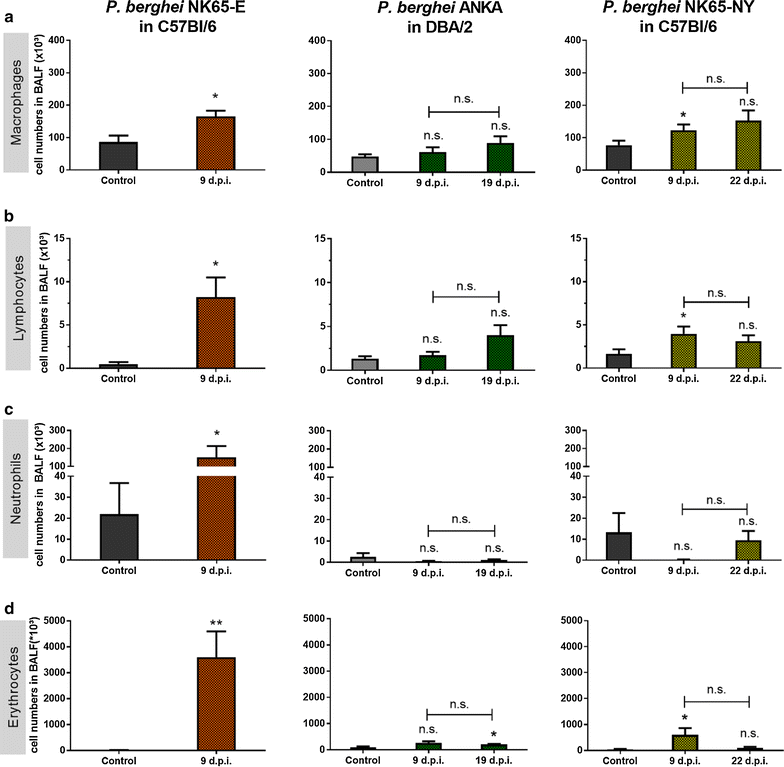
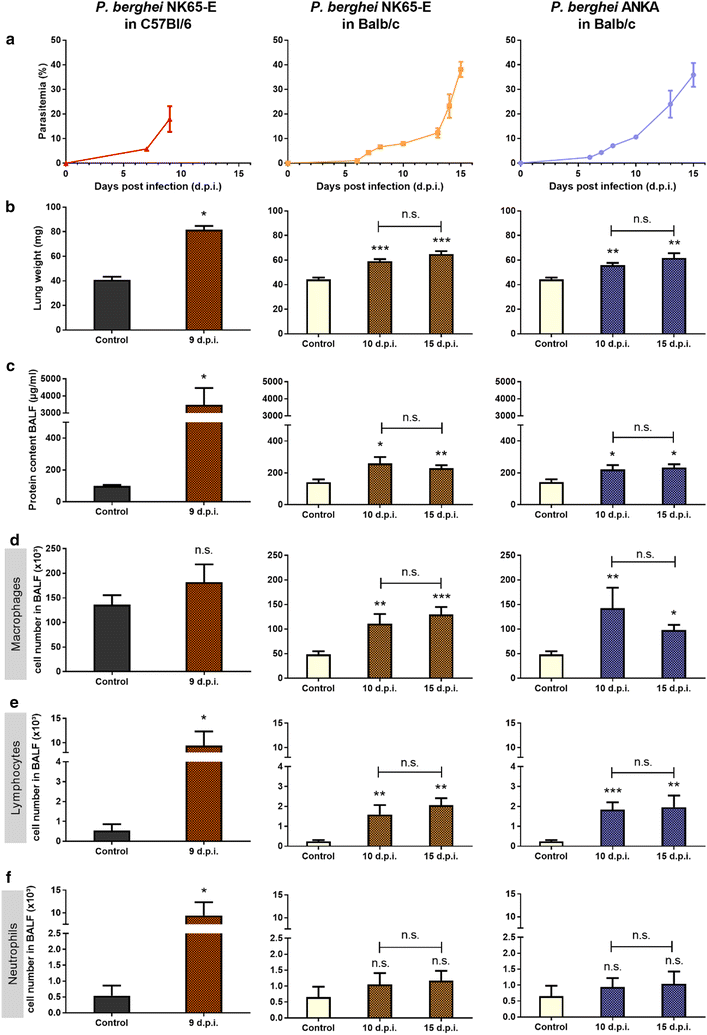
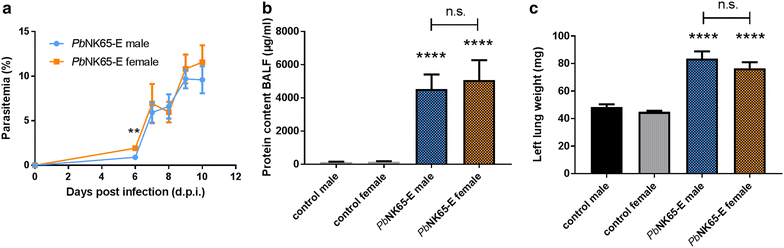
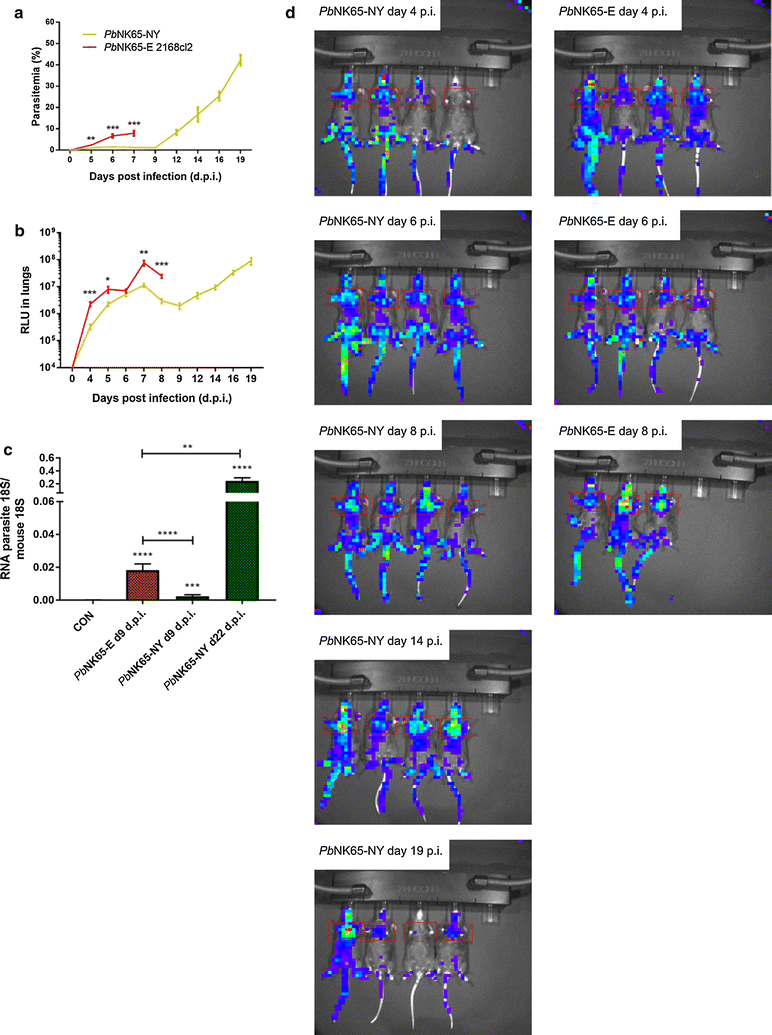
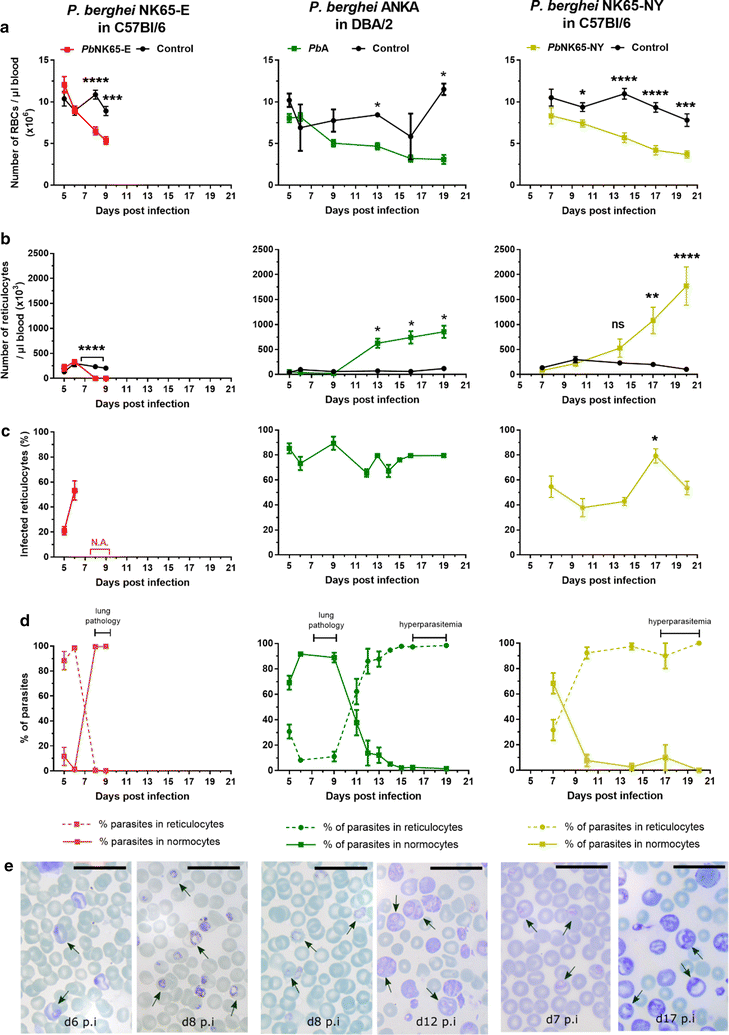
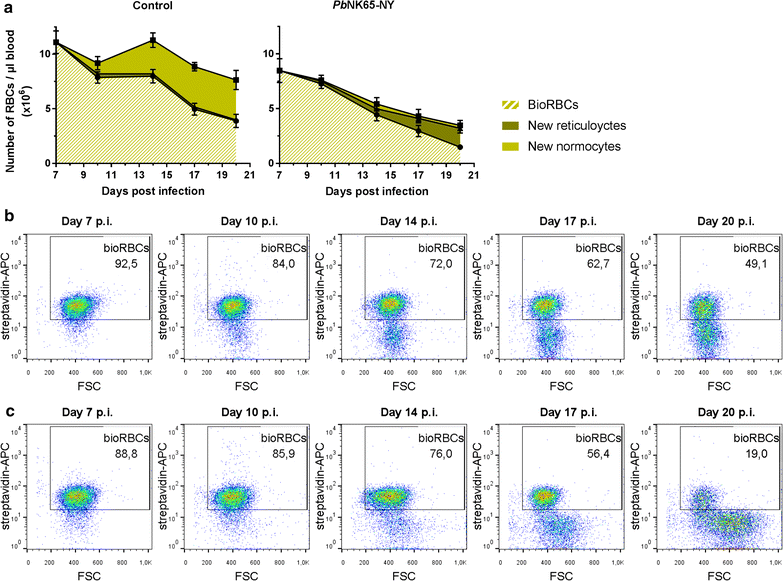
Results: Here, both host and parasite factors were demonstrated to play crucial roles in the development and severity of lung pathology. In particular, the genetic constitution of the host was an important determinant in the development of MA-ARDS. Both male and female C57BL/6, but not BALB/c, mice developed MA-ARDS when infected with P. berghei NK65-E. However, the New York line of P. berghei NK65 (NK65-NY) did not induce demonstrable MA-ARDS, despite its accumulation in the lungs and fat tissue to a similar or even higher extent as P. berghei NK65-E. These two commonly used lines of P. berghei differ in their red blood cell preference. P. berghei NK65-NY showed a stronger predilection for reticulocytes than P. berghei NK65-E and this appeared to be associated with a lower pathogenicity in the lungs. The pulmonary pathology in the C57BL/6/P. berghei NK65-E model was more pronounced than in the model with infection of DBA/2 mice with P. berghei strain ANKA. The transient lung pathology in DBA/2 mice infected with P. berghei ANKA coincided with the infection phase in which parasites mainly infected normocytes. This phase was followed by a less pathogenic phase in which P. berghei ANKA mainly infected reticulocytes.
Conclusions: The propensity of mice to develop MA-ARDS during P. berghei infection depends on both host and parasite factors and appears to correlate with RBC preference. These data provide insights in induction of MA-ARDS and may guide the choice of different mouse-parasite combinations to study lung pathology.
Keywords: Lung; Malaria-associated acute respiratory distress syndrome; Normocyte; Plasmodium berghei; Reticulocyte.
Figures
References
-
- WHO. World malaria report 2016. Geneva: World Health Organization; 2016. http://www.who.int/malaria/publications/world-malaria-report-2016/report....
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

