Attitudes of stakeholders in psychiatry towards the inclusion of children in genomic research
- PMID: 29506557
- PMCID: PMC5839067
- DOI: 10.1186/s40246-018-0144-8
Attitudes of stakeholders in psychiatry towards the inclusion of children in genomic research
Abstract
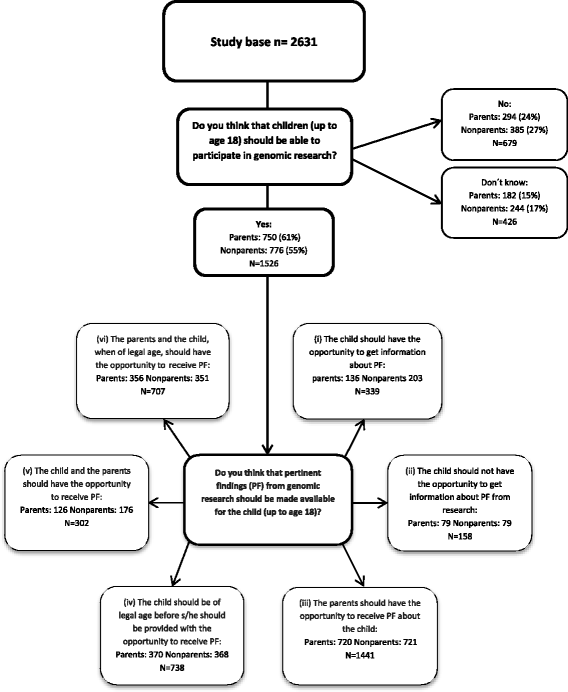
Background: Genomic sequencing of children in research raises complex ethical issues. This study aims to gain more knowledge on the attitudes towards the inclusion of children as research subjects in genomic research and towards the disclosure of pertinent and incidental findings to the parents and the child.
Methods: Qualitative data were collected from interviews with a wide range of informants: experts engaged in genomic research, clinical geneticists, persons with mental disorders, relatives, and blood donors. Quantitative data were collected from a cross-sectional web-based survey among 1227 parents and 1406 non-parents who were potential stakeholders in psychiatric genomic research.
Results: Participants generally expressed positive views on children's participation in genomic research. The informants in the qualitative interviews highlighted the age of the child as a critical aspect when disclosing genetic information. Other important aspects were the child's right to an autonomous choice, the emotional burden of knowing imposed on both the child and the parents, and the possibility of receiving beneficial clinical information regarding the future health of the child. Nevertheless, there was no consensus whether the parent or the child should receive the findings. A majority of survey stakeholders agreed that children should be able to participate in genomic research. The majority agreed that both pertinent and incidental findings should be returned to the parents and to the child when of legal age. Having children does not affect the stakeholder's attitudes towards the inclusion of children as research subjects in genomic research.
Conclusion: Our findings illustrate that both the child's right to autonomy and the parents' interest to be informed are important factors that are found valuable by the participants. In future guidelines governing children as subjects in genomic research, it would thus be essential to incorporate the child's right to an open future, including the right to receive information on adult-onset genetic disorders.
Keywords: Attitude; Child; Ethics research; Mental disorders; Minors; Whole genome sequencing.
Conflict of interest statement
Ethics approval and consent to participate
Verbal consent was obtained from each informant before the interview. All participation in the current study was voluntary. All names and personal data were removed from the transcription of the qualitative interviews in order to safeguard the anonymity of the informants.
The study was approved by the Danish Data Protection Agency (file no. 2007-58-015). As all data are based on anonymous interviews and survey information, no other ethical clearance was required according to the Committee on Health Research Ethics in the Capital Region of Denmark (file no. H-4_2013_FSP-051).
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Ethical issues in genomics research on neurodevelopmental disorders: a critical interpretive review.Hum Genomics. 2021 Mar 12;15(1):16. doi: 10.1186/s40246-021-00317-4. Hum Genomics. 2021. PMID: 33712057 Free PMC article. Review.
-
The preferences of potential stakeholders in psychiatric genomic research regarding consent procedures and information delivery.Eur Psychiatry. 2019 Jan;55:29-35. doi: 10.1016/j.eurpsy.2018.09.005. Epub 2018 Oct 29. Eur Psychiatry. 2019. PMID: 30384109
-
Stakeholders in psychiatry and their attitudes toward receiving pertinent and incident findings in genomic research.Am J Med Genet A. 2017 Oct;173(10):2649-2658. doi: 10.1002/ajmg.a.38380. Epub 2017 Aug 17. Am J Med Genet A. 2017. PMID: 28817238 Free PMC article.
-
Parents perspectives on whole genome sequencing for their children: qualified enthusiasm?J Med Ethics. 2017 Aug;43(8):535-539. doi: 10.1136/medethics-2016-103564. Epub 2016 Nov 25. J Med Ethics. 2017. PMID: 27888232
-
Ethics in child and adolescent psychiatric care: An international perspective.Int Rev Psychiatry. 2010;22(3):258-66. doi: 10.3109/09540261.2010.485979. Int Rev Psychiatry. 2010. PMID: 20528655 Review.
Cited by
-
Return of results in a global survey of psychiatric genetics researchers: practices, attitudes, and knowledge.Genet Med. 2021 Feb;23(2):298-305. doi: 10.1038/s41436-020-00986-x. Epub 2020 Oct 9. Genet Med. 2021. PMID: 33033403 Free PMC article.
-
Ethical Challenges of Germline Genetic Enhancement.Front Genet. 2019 Sep 3;10:767. doi: 10.3389/fgene.2019.00767. eCollection 2019. Front Genet. 2019. PMID: 31552088 Free PMC article. Review.
-
Return of individual research results from genomic research: A systematic review of stakeholder perspectives.PLoS One. 2021 Nov 8;16(11):e0258646. doi: 10.1371/journal.pone.0258646. eCollection 2021. PLoS One. 2021. PMID: 34748551 Free PMC article.
-
Psychiatric genomics researchers' perspectives on best practices for returning results to individual participants.Genet Med. 2020 Feb;22(2):345-352. doi: 10.1038/s41436-019-0642-7. Epub 2019 Sep 3. Genet Med. 2020. PMID: 31477844 Free PMC article.
-
Ethical issues in genomics research on neurodevelopmental disorders: a critical interpretive review.Hum Genomics. 2021 Mar 12;15(1):16. doi: 10.1186/s40246-021-00317-4. Hum Genomics. 2021. PMID: 33712057 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical