Downregulation of castor zinc finger 1 predicts poor prognosis and facilitates hepatocellular carcinoma progression via MAPK/ERK signaling
- PMID: 29506567
- PMCID: PMC5836448
- DOI: 10.1186/s13046-018-0720-8
Downregulation of castor zinc finger 1 predicts poor prognosis and facilitates hepatocellular carcinoma progression via MAPK/ERK signaling
Abstract
Background: Castor zinc finger 1 (CASZ1) plays critical roles in various biological processes and pathologic conditions, including cancer. However, the prognostic importance and biologic functions of CASZ1 in hepatocellular carcinoma (HCC) are still unclear.
Methods: qRT-PCR, western blot and immunohistochemistry analyses were used to determine CASZ1 expression in HCC samples and cell lines. The clinical significance of CASZ1 was assessed in two independent study cohorts containing 232 patients with HCC. A series of in vitro and in vivo experiments were performed to explore the role and molecular mechanism of CASZ1 in HCC progression.
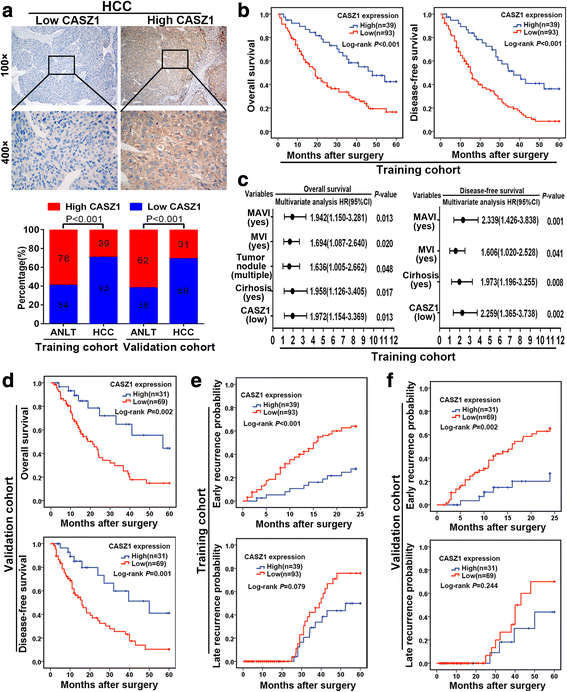
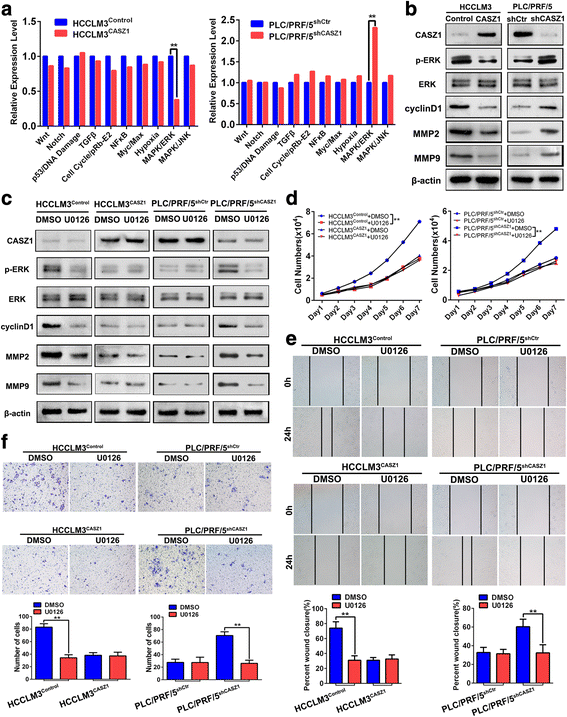
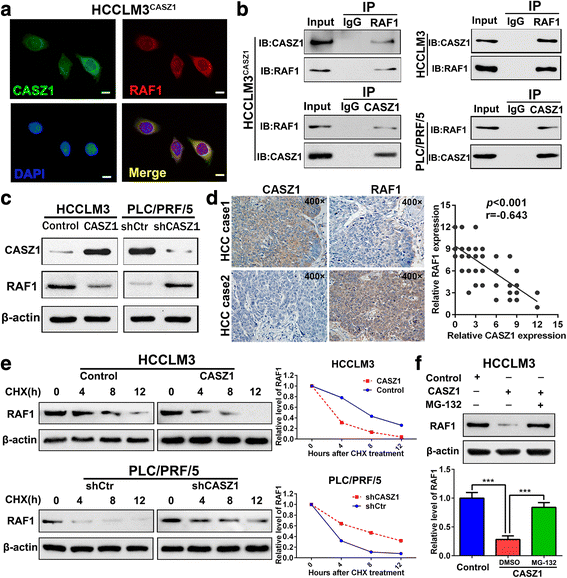
Results: Here we report that CASZ1 expression was downregulated in HCC tissues and cell lines. Low CASZ1 expression was closely correlated with aggressive clinicopathological features, poor clinical outcomes and early recurrence of HCC patients. Moreover, overexpression of CASZ1 in HCCLM3 cells significantly inhibited cell proliferation, migration, invasion in vitro and tumor growth and metastasis in vivo, whereas silencing CASZ1 significantly enhanced the above abilities of PLC/PRF/5 cells. Further mechanism study indicated that these phenotypic changes were mediated by MAPK/ERK signaling pathway and involved altered expression of MMP2, MMP9 and cyclinD1. Finally, we proved that CASZ1 exerted its tumor-suppressive effect by directly interacting with RAF1 and reducing the protein stability of RAF1.
Conclusions: Our study for the first time demonstrated that CASZ1 is a tumor suppressor in HCC, which may serve as a novel prognostic predictor and therapeutic target for HCC patients.
Keywords: Castor zinc finger 1; Hepatocellular carcinoma; MAPK/ERK; Progression; RAF1.
Conflict of interest statement
Ethics approval and consent to participate
The studies were approved by the Ethics Committee of Xiangya Hospital of Central South University and Affiliated Cancer Hospital of Xiangya School of Medicine. Written informed consent was obtained from all patients. Animal experiments were approved by the Institutional Animal Care and Use Committee of Central South University.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
MeSH terms
Substances
Grants and funding
- 81172018, 81272395/National Natural Science Foundation of China
- 81330057/Key Project of National Nature Science Foundation of China
- 2016YFC0902400/National Key R&D Program of China
- 20130162130007/Specialized Research Fund for Doctoral Program of Higher Education of China
- 2014zzts088/Fundamental Research Funds for the Central Universities of Central South University
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

