High-flow nasal oxygen vs. standard oxygen therapy in immunocompromised patients with acute respiratory failure: study protocol for a randomized controlled trial
- PMID: 29506579
- PMCID: PMC5836389
- DOI: 10.1186/s13063-018-2492-z
High-flow nasal oxygen vs. standard oxygen therapy in immunocompromised patients with acute respiratory failure: study protocol for a randomized controlled trial
Abstract
Background: Acute respiratory failure (ARF) is the leading reason for intensive care unit (ICU) admission in immunocompromised patients. High-flow nasal oxygen (HFNO) therapy is an alternative to standard oxygen. By providing warmed and humidified gas, HFNO allows the delivery of higher flow rates via nasal cannula devices, with FiO2 values of nearly 100%. Benefits include alleviation of dyspnea and discomfort, decreased respiratory distress and decreased mortality in unselected patients with acute hypoxemic respiratory failure. However, in preliminary reports, HFNO benefits are controversial in immunocompromised patients in whom it has never been properly evaluated.
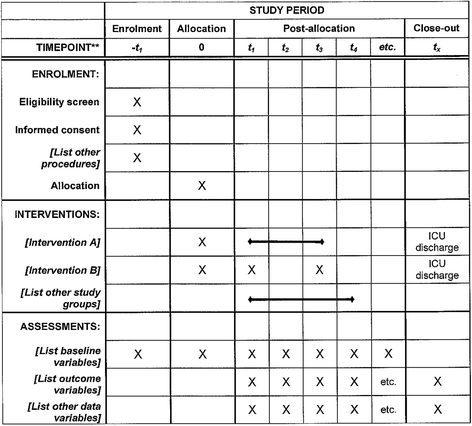
Methods/design: This is a multicenter, open-label, randomized controlled superiority trial in 30 intensive care units, part of the Groupe de Recherche Respiratoire en Réanimation Onco-Hématologique (GRRR-OH). Inclusion criteria will be: (1) adults, (2) known immunosuppression, (3) ARF, (4) oxygen therapy ≥ 6 L/min, (5) written informed consent from patient or proxy. Exclusion criteria will be: (1) imminent death (moribund patient), (2) no informed consent, (3) hypercapnia (PaCO2 ≥ 50 mmHg), (4) isolated cardiogenic pulmonary edema, (5) pregnancy or breastfeeding, (6) anatomical factors precluding insertion of a nasal cannula, (7) no coverage by the French statutory healthcare insurance system, and (8) post-surgical setting from day 1 to day 6 (patients with ARF occurring after day 6 of surgery can be included). The primary outcome measure is day-28 mortality. Secondary outcomes are intubation rate, comfort, dyspnea, respiratory rate, oxygenation, ICU length of stay, and ICU-acquired infections. Based on an expected 30% mortality rate in the standard oxygen group, and 20% in the HFNO group, error rate set at 5%, and a statistical power at 90%, 389 patients are required in each treatment group (778 patients overall). Recruitment period is estimated at 30 months, with 28 days of additional follow-up for the last included patient.
Discussion: The HIGH study will be the largest multicenter, randomized controlled trial seeking to demonstrate that survival benefits from HFNO reported in unselected patients also apply to a large immunocompromised population.
Trial registration: ClinicalTrials.gov, ID: NCT02739451 . Registered on 15 April 2016.
Keywords: Acute respiratory failure; High-flow oxygen; Immunocompromised Hematology; Immunosuppression; Intubation; Mortality; Oxygen.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by the IRB of the St. Louis Hospital. All patients or relatives provided signed informed consent.
Consent for publication
All authors consent to see this protocol article published. All have given input on the submitted version and approved it.
Competing interests
None of the authors has any conflict of interest in relation with this study. The institutions of Elie Azoulay, Samir Jaber, Alexandre Demoule and Virginie Lemiale have received scientific support from Fisher & Payckle outside this study.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
-
- Dumas G, Geri G, Montlahuc C, et al. Outcomes in critically ill patients with systemic rheumatic disease: a multicenter study. Chest. 2015;2015(21):14–3098. - PubMed
-
- Azoulay E, Pene F, Darmon M, et al. Managing critically Ill hematology patients: time to think differently. Blood Rev. 2015;2015(26):00030–00032. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous