The REstart or STop Antithrombotics Randomised Trial (RESTART) after stroke due to intracerebral haemorrhage: study protocol for a randomised controlled trial
- PMID: 29506580
- PMCID: PMC5838871
- DOI: 10.1186/s13063-018-2542-6
The REstart or STop Antithrombotics Randomised Trial (RESTART) after stroke due to intracerebral haemorrhage: study protocol for a randomised controlled trial
Abstract
Background: For adults surviving stroke due to spontaneous (non-traumatic) intracerebral haemorrhage (ICH) who had taken an antithrombotic (i.e. anticoagulant or antiplatelet) drug for the prevention of vaso-occlusive disease before the ICH, it is unclear whether starting antiplatelet drugs results in an increase in the risk of recurrent ICH or a beneficial net reduction of all serious vascular events compared to avoiding antiplatelet drugs.
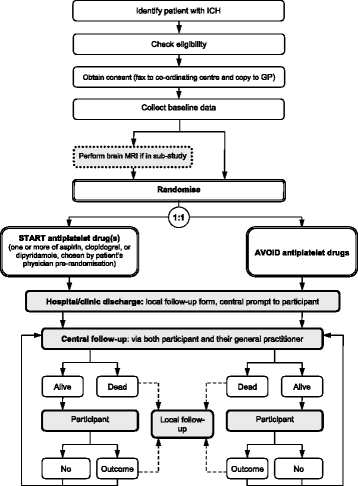
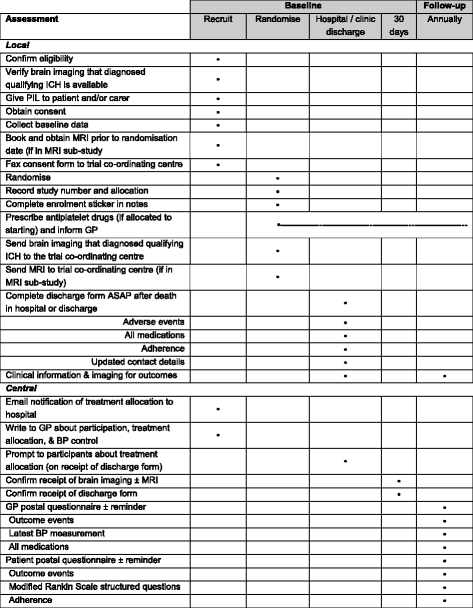
Methods/design: The REstart or STop Antithrombotics Randomised Trial (RESTART) is an investigator-led, randomised, open, assessor-blind, parallel-group, randomised trial comparing starting versus avoiding antiplatelet drugs for adults surviving antithrombotic-associated ICH at 122 hospital sites in the United Kingdom. RESTART uses a central, web-based randomisation system using a minimisation algorithm, with 1:1 treatment allocation to which central research staff are masked. Central follow-up includes annual postal or telephone questionnaires to participants and their general (family) practitioners, with local provision of information about adverse events and outcome events. The primary outcome is recurrent symptomatic ICH. The secondary outcomes are: symptomatic haemorrhagic events; symptomatic vaso-occlusive events; symptomatic stroke of uncertain type; other fatal events; modified Rankin Scale score; adherence to antiplatelet drug(s). The magnetic resonance imaging (MRI) sub-study involves the conduct of brain MRI according to a standardised imaging protocol before randomisation to investigate heterogeneity of treatment effect according to the presence of brain microbleeds. Recruitment began on 22 May 2013. The target sample size is at least 720 participants in the main trial (at least 550 in the MRI sub-study).
Discussion: Final results of RESTART will be analysed and disseminated in 2019.
Trial registration: ISRCTN71907627 ( www.isrctn.com/ISRCTN71907627 ). Prospectively registered on 25 April 2013.
Keywords: Antiplatelet therapy; Intracerebral haemorrhage; Randomised controlled trial; Secondary prevention; Stroke.
Conflict of interest statement
Ethics approval and consent to participate
The Scotland A Research Ethics Committee approved RESTART before recruitment began (reference 12/SS/0138). Collaborators who have received GCP and RESTART-specific training and are included in a delegation log obtain written informed consent from participants or their legal representative or nearest relative.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Antithrombotic Trialists Collaboration. Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, Buring J, Hennekens C, Kearney P, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373(9678):1849–1860. doi: 10.1016/S0140-6736(09)60503-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous