A novel method to generate T-cell receptor-deficient chimeric antigen receptor T cells
- PMID: 29507075
- PMCID: PMC5851418
- DOI: 10.1182/bloodadvances.2017012823
A novel method to generate T-cell receptor-deficient chimeric antigen receptor T cells
Abstract
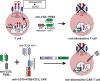
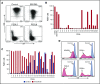
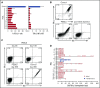
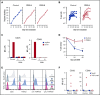
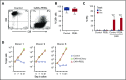
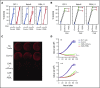
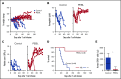
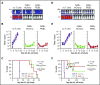
Practical methods are needed to increase the applicability and efficacy of chimeric antigen receptor (CAR) T-cell therapies. Using donor-derived CAR-T cells is attractive, but expression of endogenous T-cell receptors (TCRs) carries the risk for graft-versus-host-disease (GVHD). To remove surface TCRαβ, we combined an antibody-derived single-chain variable fragment specific for CD3ε with 21 different amino acid sequences predicted to retain it intracellularly. After transduction in T cells, several of these protein expression blockers (PEBLs) colocalized intracellularly with CD3ε, blocking surface CD3 and TCRαβ expression. In 25 experiments, median TCRαβ expression in T lymphocytes was reduced from 95.7% to 25.0%; CD3/TCRαβ cell depletion yielded virtually pure TCRαβ-negative T cells. Anti-CD3ε PEBLs abrogated TCRαβ-mediated signaling, without affecting immunophenotype or proliferation. In anti-CD3ε PEBL-T cells, expression of an anti-CD19-41BB-CD3ζ CAR induced cytokine secretion, long-term proliferation, and CD19+ leukemia cell killing, at rates meeting or exceeding those of CAR-T cells with normal CD3/TCRαβ expression. In immunodeficient mice, anti-CD3ε PEBL-T cells had markedly reduced GVHD potential; when transduced with anti-CD19 CAR, these T cells killed engrafted leukemic cells. PEBL blockade of surface CD3/TCRαβ expression is an effective tool to prepare allogeneic CAR-T cells. Combined PEBL and CAR expression can be achieved in a single-step procedure, is easily adaptable to current cell manufacturing protocols, and can be used to target other T-cell molecules to further enhance CAR-T-cell therapies.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: T.K., Y.T.P., and D.C. are coinventors in patent applications describing the technologies used. D.W. declares no competing financial interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

