VASCULAR PLANT ONE-ZINC FINGER1 (VOZ1) and VOZ2 Interact with CONSTANS and Promote Photoperiodic Flowering Transition
- PMID: 29507119
- PMCID: PMC5884617
- DOI: 10.1104/pp.17.01562
VASCULAR PLANT ONE-ZINC FINGER1 (VOZ1) and VOZ2 Interact with CONSTANS and Promote Photoperiodic Flowering Transition
Abstract
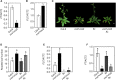
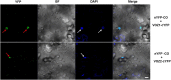
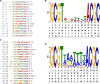
In plants, endogenous and environmental signals such as light control the timing of the transition to flowering. Two phytochrome B-interacting transcription factors, VASCULAR PLANT ONE-ZINC FINGER1 (VOZ1) and VOZ2, redundantly promote flowering in Arabidopsis (Arabidopsis thaliana). In the voz1 voz2 mutant, the expression of FLOWERING LOCUS C (FLC) was up-regulated and that of FLOWERING LOCUS T (FT) was down-regulated, which was proposed to be the cause of late flowering in voz1 voz2 However, the detailed mechanism by which the VOZ genes promote flowering is not well understood. Here, we show that neither the reduced FT expression nor the late-flowering phenotype of voz1 voz2 is suppressed in the voz1 voz2 flc triple mutant. Genetic interaction experiments between voz1 voz2 and constans-2 (co-2) mutants reveal that the VOZs and CO work in the same genetic pathway. Using in vitro pull-down, electrophoretic mobility shift, and bimolecular fluorescence complementation assays, we show that VOZ1 and VOZ2 interact with CO. The voz1 voz2 35S::CO:YFP plants show suppression of the early-flowering phenotype induced by CO overexpression, suggesting that CO requires VOZ for the induction of flowering. Determination of the VOZ consensus-binding site followed by genome-wide sequence analysis failed to identify any VOZ-binding sites near known flowering time genes. Together, these results indicate that the VOZ genes regulate flowering primarily through the photoperiod pathway, independent of FLC, and suggest that VOZs modulate CO function to promote flowering.
© 2018 American Society of Plant Biologists. All Rights Reserved.
Figures




Similar articles
-
The PHYB-FOF2-VOZ2 module functions to fine-tune flowering in response to changes in light quality by modulating FLC expression in Arabidopsis.Plant Commun. 2024 Jul 8;5(7):100922. doi: 10.1016/j.xplc.2024.100922. Epub 2024 Apr 14. Plant Commun. 2024. PMID: 38616490 Free PMC article.
-
VASCULAR PLANT ONE-ZINC FINGER1 and VOZ2 repress the FLOWERING LOCUS C clade members to control flowering time in Arabidopsis.Biosci Biotechnol Biochem. 2014;78(11):1850-5. doi: 10.1080/09168451.2014.932670. Epub 2014 Jul 10. Biosci Biotechnol Biochem. 2014. PMID: 25351333
-
The phytochrome-interacting vascular plant one-zinc finger1 and VOZ2 redundantly regulate flowering in Arabidopsis.Plant Cell. 2012 Aug;24(8):3248-63. doi: 10.1105/tpc.112.101915. Epub 2012 Aug 17. Plant Cell. 2012. PMID: 22904146 Free PMC article.
-
Circadian clock and photoperiodic response in Arabidopsis: from seasonal flowering to redox homeostasis.Biochemistry. 2015 Jan 20;54(2):157-70. doi: 10.1021/bi500922q. Epub 2014 Dec 30. Biochemistry. 2015. PMID: 25346271 Free PMC article. Review.
-
The control of flowering in time and space.J Exp Bot. 2006;57(13):3415-8. doi: 10.1093/jxb/erl159. Epub 2006 Sep 27. J Exp Bot. 2006. PMID: 17005922 Review.
Cited by
-
Vascular Plant One-Zinc-Finger (VOZ) Transcription Factors Are Positive Regulators of Salt Tolerance in Arabidopsis.Int J Mol Sci. 2018 Nov 23;19(12):3731. doi: 10.3390/ijms19123731. Int J Mol Sci. 2018. PMID: 30477148 Free PMC article.
-
Regulation of Flowering Time by Environmental Factors in Plants.Plants (Basel). 2023 Oct 25;12(21):3680. doi: 10.3390/plants12213680. Plants (Basel). 2023. PMID: 37960036 Free PMC article. Review.
-
B-box transcription factor 28 regulates flowering by interacting with constans.Sci Rep. 2020 Oct 20;10(1):17789. doi: 10.1038/s41598-020-74445-7. Sci Rep. 2020. PMID: 33082412 Free PMC article.
-
Expression of a maize SOC1 gene enhances soybean yield potential through modulating plant growth and flowering.Sci Rep. 2021 Jun 17;11(1):12758. doi: 10.1038/s41598-021-92215-x. Sci Rep. 2021. PMID: 34140602 Free PMC article.
-
The PHYB-FOF2-VOZ2 module functions to fine-tune flowering in response to changes in light quality by modulating FLC expression in Arabidopsis.Plant Commun. 2024 Jul 8;5(7):100922. doi: 10.1016/j.xplc.2024.100922. Epub 2024 Apr 14. Plant Commun. 2024. PMID: 38616490 Free PMC article.
References
-
- Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y, Ichinoki H, Notaguchi M, Goto K, Araki T (2005) FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309: 1052–1056 - PubMed
-
- An H, Roussot C, Suárez-López P, Corbesier L, Vincent C, Piñeiro M, Hepworth S, Mouradov A, Justin S, Turnbull C, et al. (2004) CONSTANS acts in the phloem to regulate a systemic signal that induces photoperiodic flowering of Arabidopsis. Development 131: 3615–3626 - PubMed
-
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 - PubMed
-
- Corbesier L, Gadisseur I, Silvestre G, Jacqmard A, Bernier G (1996) Design in Arabidopsis thaliana of a synchronous system of floral induction by one long day. Plant J 9: 947–952 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

