Single-molecule analysis of phospholipid scrambling by TMEM16F
- PMID: 29507235
- PMCID: PMC5866571
- DOI: 10.1073/pnas.1717956115
Single-molecule analysis of phospholipid scrambling by TMEM16F
Abstract
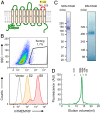
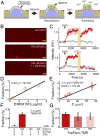
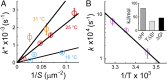
Transmembrane protein 16F (TMEM16F) is a Ca2+-dependent phospholipid scramblase that translocates phospholipids bidirectionally between the leaflets of the plasma membrane. Phospholipid scrambling of TMEM16F causes exposure of phosphatidylserine in activated platelets to induce blood clotting and in differentiated osteoblasts to promote bone mineralization. Despite the importance of TMEM16F-mediated phospholipid scrambling in various biological reactions, the fundamental features of the scrambling reaction remain elusive due to technical difficulties in the preparation of a platform for assaying scramblase activity in vitro. Here, we established a method to express and purify mouse TMEM16F as a dimeric molecule by constructing a stable cell line and developed a microarray containing membrane bilayers with asymmetrically distributed phospholipids as a platform for single-molecule scramblase assays. The purified TMEM16F was integrated into the microarray, and monitoring of phospholipid translocation showed that a single TMEM16F molecule transported phospholipids nonspecifically between the membrane bilayers in a Ca2+-dependent manner. Thermodynamic analysis of the reaction indicated that TMEM16F transported 4.5 × 104 lipids per second at 25 °C, with an activation free energy of 47 kJ/mol. These biophysical features were similar to those observed with channels, which transport substrates by facilitating diffusion, and supported the stepping-stone model for the TMEM16F phospholipid scramblase.
Keywords: TMEM16F; membrane protein; microsystem; phospholipid scrambling; single-molecule analysis.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Leventis PA, Grinstein S. The distribution and function of phosphatidylserine in cellular membranes. Annu Rev Biophys. 2010;39:407–427. - PubMed
-
- Balasubramanian K, Schroit AJ. Aminophospholipid asymmetry: A matter of life and death. Annu Rev Physiol. 2003;65:701–734. - PubMed
-
- Segawa K, et al. Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science. 2014;344:1164–1168. - PubMed
-
- Segawa K, Nagata S. An apoptotic ‘eat me’ signal: Phosphatidylserine exposure. Trends Cell Biol. 2015;25:639–650. - PubMed
-
- Suzuki J, Umeda M, Sims PJ, Nagata S. Calcium-dependent phospholipid scrambling by TMEM16F. Nature. 2010;468:834–838. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

