Surface chemical heterogeneity modulates silica surface hydration
- PMID: 29507240
- PMCID: PMC5866604
- DOI: 10.1073/pnas.1722263115
Surface chemical heterogeneity modulates silica surface hydration
Abstract
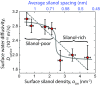
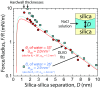
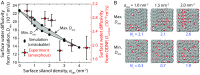
An in-depth knowledge of the interaction of water with amorphous silica is critical to fundamental studies of interfacial hydration water, as well as to industrial processes such as catalysis, nanofabrication, and chromatography. Silica has a tunable surface comprising hydrophilic silanol groups and moderately hydrophobic siloxane groups that can be interchanged through thermal and chemical treatments. Despite extensive studies of silica surfaces, the influence of surface hydrophilicity and chemical topology on the molecular properties of interfacial water is not well understood. In this work, we controllably altered the surface silanol density, and measured surface water diffusivity using Overhauser dynamic nuclear polarization (ODNP) and complementary silica-silica interaction forces across water using a surface forces apparatus (SFA). The results show that increased silanol density generally leads to slower water diffusivity and stronger silica-silica repulsion at short aqueous separations (less than ∼4 nm). Both techniques show sharp changes in hydration properties at intermediate silanol densities (2.0-2.9 nm-2). Molecular dynamics simulations of model silica-water interfaces corroborate the increase in water diffusivity with silanol density, and furthermore show that even on a smooth and crystalline surface at a fixed silanol density, adjusting the spatial distribution of silanols results in a range of surface water diffusivities spanning ∼10%. We speculate that a critical silanol cluster size or connectivity parameter could explain the sharp transition in our results, and can modulate wettability, colloidal interactions, and surface reactions, and thus is a phenomenon worth further investigation on silica and chemically heterogeneous surfaces.
Keywords: hydration dynamics; hydrophobicity; silica; surface forces; water.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Iler RK. The Chemistry of Silica. Wiley; New York: 1979. pp. 3–10, 624–648.
-
- Zhuravlev LT. The surface chemistry of amorphous silica. Zhuravlev model. Colloids Surf A Physicochem Eng Asp. 2000;173:1–38.
-
- Ek S, Root A, Peussa M, Niinistö L. Determination of the hydroxyl group content in silica by thermogravimetry and a comparison with 1H MAS NMR results. Thermochim Acta. 2001;379:201–212.
-
- Gallas J-P, et al. Quantification of water and silanol species on various silicas by coupling IR spectroscopy and in-situ thermogravimetry. Langmuir. 2009;25:5825–5834. - PubMed
-
- Christy AA, Egeberg PK. Quantitative determination of surface silanol groups in silicagel by deuterium exchange combined with infrared spectroscopy and chemometrics. Analyst (Lond) 2005;130:738–744. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

