Uncovering the balance of forces driving microtubule aster migration in C. elegans zygotes
- PMID: 29507295
- PMCID: PMC5838244
- DOI: 10.1038/s41467-018-03118-x
Uncovering the balance of forces driving microtubule aster migration in C. elegans zygotes
Abstract
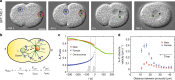
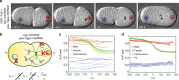
Microtubule asters must be positioned precisely within cells. How forces generated by molecular motors such as dynein are integrated in space and time to enable such positioning remains unclear. In particular, whereas aster movements depend on the drag caused by cytoplasm viscosity, in vivo drag measurements are lacking, precluding a thorough understanding of the mechanisms governing aster positioning. Here, we investigate this fundamental question during the migration of asters and pronuclei in C. elegans zygotes, a process essential for the mixing of parental genomes. Detailed quantification of these movements using the female pronucleus as an in vivo probe establish that the drag coefficient of the male-asters complex is approximately five times that of the female pronucleus. Further analysis of embryos lacking cortical dynein, the connection between asters and male pronucleus, or the male pronucleus altogether, uncovers the balance of dynein-driven forces that accurately position microtubule asters in C. elegans zygotes.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
The branched actin nucleator Arp2/3 promotes nuclear migrations and cell polarity in the C. elegans zygote.Dev Biol. 2011 Sep 15;357(2):356-69. doi: 10.1016/j.ydbio.2011.07.008. Epub 2011 Jul 18. Dev Biol. 2011. PMID: 21798253 Free PMC article.
-
Computer simulations reveal mechanisms that organize nuclear dynein forces to separate centrosomes.Mol Biol Cell. 2017 Nov 7;28(23):3165-3170. doi: 10.1091/mbc.E16-12-0823. Epub 2017 Jul 12. Mol Biol Cell. 2017. PMID: 28701341 Free PMC article.
-
Cytoplasmic dynein is required for distinct aspects of MTOC positioning, including centrosome separation, in the one cell stage Caenorhabditis elegans embryo.J Cell Biol. 1999 Oct 4;147(1):135-50. doi: 10.1083/jcb.147.1.135. J Cell Biol. 1999. PMID: 10508861 Free PMC article.
-
Physical Limits on the Precision of Mitotic Spindle Positioning by Microtubule Pushing forces: Mechanics of mitotic spindle positioning.Bioessays. 2017 Nov;39(11):10.1002/bies.201700122. doi: 10.1002/bies.201700122. Epub 2017 Sep 28. Bioessays. 2017. PMID: 28960439 Free PMC article. Review.
-
Growth, interaction, and positioning of microtubule asters in extremely large vertebrate embryo cells.Cytoskeleton (Hoboken). 2012 Oct;69(10):738-50. doi: 10.1002/cm.21050. Epub 2012 Aug 20. Cytoskeleton (Hoboken). 2012. PMID: 22786885 Free PMC article. Review.
Cited by
-
Size- and position-dependent cytoplasm viscoelasticity through hydrodynamic interactions with the cell surface.Proc Natl Acad Sci U S A. 2023 Feb 28;120(9):e2216839120. doi: 10.1073/pnas.2216839120. Epub 2023 Feb 21. Proc Natl Acad Sci U S A. 2023. PMID: 36802422 Free PMC article.
-
Mechanical Regulation of Nuclear Translocation in Migratory Neurons.Front Cell Dev Biol. 2020 Mar 12;8:150. doi: 10.3389/fcell.2020.00150. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32226788 Free PMC article. Review.
-
Gamete Nuclear Migration in Animals and Plants.Front Plant Sci. 2019 Apr 24;10:517. doi: 10.3389/fpls.2019.00517. eCollection 2019. Front Plant Sci. 2019. PMID: 31068960 Free PMC article. Review.
-
Contribution of cytoplasm viscoelastic properties to mitotic spindle positioning.Proc Natl Acad Sci U S A. 2022 Feb 22;119(8):e2115593119. doi: 10.1073/pnas.2115593119. Proc Natl Acad Sci U S A. 2022. PMID: 35169074 Free PMC article.
-
Dynein-dependent collection of membranes defines the architecture and position of microtubule asters in isolated, geometrically confined volumes of cell-free extracts.Mol Biol Cell. 2022 Sep 15;33(11):br20. doi: 10.1091/mbc.E22-03-0074. Epub 2022 Aug 17. Mol Biol Cell. 2022. PMID: 35976715 Free PMC article.
References
-
- Reinsch S, Gönczy P. Mechanisms of nuclear positioning. J. Cell Sci. 1998;111:2283–2295. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

