Allogeneic dendritic cells stimulated with antibodies against HLA class II polarize naive T cells in a follicular helper phenotype
- PMID: 29507364
- PMCID: PMC5838222
- DOI: 10.1038/s41598-018-22391-w
Allogeneic dendritic cells stimulated with antibodies against HLA class II polarize naive T cells in a follicular helper phenotype
Abstract
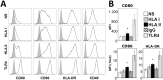
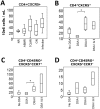
Follicular helper T cells (Tfh) are crucial for the production of high-affinity antibodies, such as alloantibodies, by providing the signals for B-cell proliferation and differentiation. Here, we demonstrate that human allogeneic dendritic cells (DC) stimulated with antibodies against HLA class II antigens preferentially differentiate human naive CD4+ T cells into Tfh cells. Following coculture with DCs treated with these antibodies, CD4+ T cells expressed CXCR5, ICOS, IL-21, Bcl-6 and phosphorylated STAT3. Blockade of IL-21 abrogated Bcl-6, while addition of the IL-12p40 subunit to the coculture increased CXCR5, Bcl-6, phosphorylated STAT3 and ICOS, indicating that they were both involved in Tfh polarization. We further phenotyped the peripheral T cells in a cohort of 55 kidney transplant recipients. Patients with anti-HLA-II donor-specific antibodies (DSA) presented higher blood counts of circulating Tfh cells than those with anti-HLA-I DSAs. Moreover, there was a predominance of lymphoid aggregates containing Tfh cells in biopsies from patients with antibody-mediated rejection and anti-HLA-II DSAs. Collectively, these data suggest that alloantibodies against HLA class II specifically promote the differentiation of naive T cells to Tfh cells following contact with DCs, a process that might appear in situ in human allografts and constitutes a therapeutic target.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Sellares J, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12:388–399. doi: 10.1111/j.1600-6143.2011.03840.x. - DOI - PubMed
-
- Sellares J, et al. Molecular diagnosis of antibody-mediated rejection in human kidney transplants. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13:971–983. doi: 10.1111/ajt.12150. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

