Azacitidine-induced pyoderma gangrenosum at injection sites in a patient with myelodysplastic syndrome
- PMID: 29507503
- PMCID: PMC5832284
- DOI: 10.3747/co.25.3779
Azacitidine-induced pyoderma gangrenosum at injection sites in a patient with myelodysplastic syndrome
Abstract
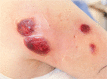
Pyoderma gangrenosum (pg) is a rare neutrophilic dermatosis characterized by painful necrotic ulceration affecting preferentially the lower extremities. Diagnosis is challenging, and a thorough workup (including biopsy) is required. In this case report, we describe a 67-year-old patient with a diagnosis of myelodysplastic syndrome (mds) who developed fever and pg two days after the first cycle of subcutaneous azacitidine (Vidaza; Celgene Corporation, Summit, NJ, USA). On physical examination, the patient had four erythematous plaques at sites of subcutaneous injections of azacitidine on the arms, as well as three other plaques in proximity. A skin biopsy demonstrated a dense neutrophilic interstitial infiltrate in the dermis. After the diagnosis of pg, prednisone 1 mg/kg was started and the fever subsided rapidly. This was followed by the resolution of the cutaneous lesions. Changing the route of administration of azacitidine from subcutaneous to intravenous and adding a daily dose of prednisone during the treatment allowed the patient to receive a total of 10 cycles of azacitidine. This is the second case reported in the literature. Because azacitidine is frequently used in mds and acute myeloid leukemia, clinicians should be aware of this rare cutaneous adverse event. Our approach can be used to avoid the recurrence of pg when continuing azacitidine treatment.
Keywords: Azacitidine; myelodysplastic syndrome; neutrophilic dermatosis; pyoderma gangrenosum.
Figures

References
-
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10(3):223–32. doi: 10.1016/S1470-2045(09)70003-8. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

