A novel, homozygous mutation in desert hedgehog (DHH) in a 46, XY patient with dysgenetic testes presenting with primary amenorrhoea: a case report
- PMID: 29507583
- PMCID: PMC5834851
- DOI: 10.1186/s13633-018-0056-3
A novel, homozygous mutation in desert hedgehog (DHH) in a 46, XY patient with dysgenetic testes presenting with primary amenorrhoea: a case report
Abstract
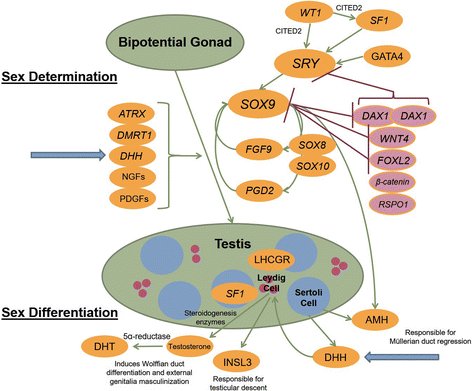
Background: Desert hedgehog (DHH) mutations have been described in only a limited number of individuals with 46, XY disorders of sex development (DSD) presenting as either partial or complete gonadal dysgenesis. Gonadal tumours and peripheral neuropathy have been associated with DHH mutations. Herein we report a novel, homozygous mutation of DHH identified through a targeted, massively parallel sequencing (MPS) DSD panel, in a patient presenting with partial gonadal dysgenesis. This novel mutation is two amino acids away from a previously described mutation in a patient who presented with complete gonadal dysgenesis. Adding to the complexity of work-up, our patient also expressed gender identity concern.
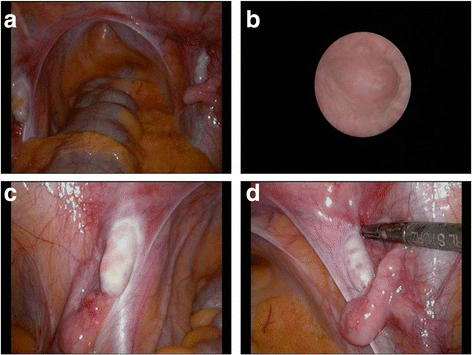
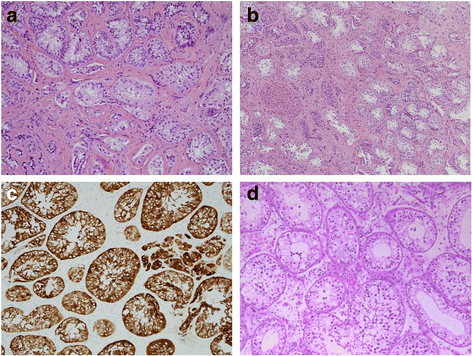
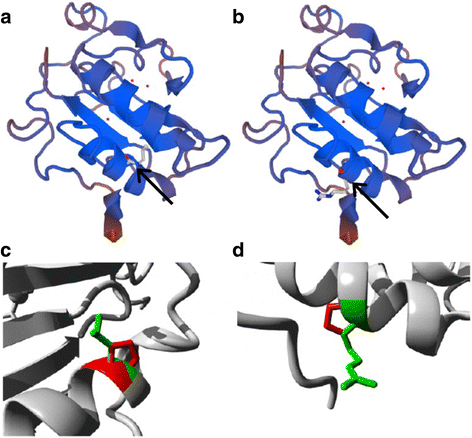
Case presentation: A 14-year-old, phenotypic female presented with primary amenorrhoea and absent secondary sex characteristics. Investigations revealed elevated gonadotrophins with low oestradiol, testosterone of 0.6 nmol/L and a 46, XY karyotype. Müllerian structures were not seen on pelvic ultrasound or laparoscopically and gonadal biopsies demonstrated dysgenetic testes without neoplasia (partial gonadal dysgenesis). The patient expressed gender identity confusion upon initial notification of investigation findings. Formal psychiatric evaluation excluded gender dysphoria. Genetic analysis was performed using a targeted, MPS DSD panel of 64 diagnostic and 927 research candidate genes. This identified a novel, homozygous mutation in exon 2 of DHH (DHH:NM_021044:exon2:c.G491C:p.R164P). With this finding our patient was screened for the possibility of peripheral neuropathy which was not evident clinically nor on investigation. She was commenced on oestrogen for pubertal induction.
Conclusion: The evaluation of patients with DSD is associated with considerable psychological distress. Targeted MPS enables an affordable and efficient method for diagnosis of 46, XY DSD cases. Identifying a genetic diagnosis may inform clinical management and in this case directed screening for peripheral neuropathy. In addition to the structural location of the mutation other interacting factors may influence phenotypic expression in homozygous DHH mutations.
Keywords: DHH; Desert hedgehog; Disorder of sex development; Gonadal dysgenesis; Massively parallel sequencing.
Conflict of interest statement
The Princess Margaret Hospital for Children Ethics Committee has given approval for this case report.Written informed consent was obtained from the patient and the patient’s legal guardian for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Mendonca BB, Domenice S, Arnhold IJ, Costa EM. 46, XY disorders of sex development (DSD) Clin Endocrinol. 2009;70:173–187. - PubMed
-
- Rey R, Josso N, Racine C. In: Sexual Differentiation. De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R, New M, Purnell J, Rebar R, Singer F, Vinik A, editors. South Dartmouth (MA): Endotext; 2000.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

