Serum exosomes can restore cellular function in vitro and be used for diagnosis in dysferlinopathy
- PMID: 29507617
- PMCID: PMC5835933
- DOI: 10.7150/thno.22856
Serum exosomes can restore cellular function in vitro and be used for diagnosis in dysferlinopathy
Erratum in
-
Erratum: Serum exosomes can restore cellular function in vitro and be used for diagnosis in dysferlinopathy: Erratum.Theranostics. 2021 Jun 8;11(15):7616-7617. doi: 10.7150/thno.62181. eCollection 2021. Theranostics. 2021. PMID: 34158870 Free PMC article.
Abstract
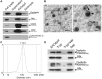
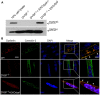
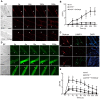
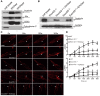
Purpose: It is challenging to deliver the full-length dysferlin gene or protein to restore cellular functions of dysferlin-deficient (DYSF-/-) myofibres in dysferlinopathy, a disease caused by the absence of dysferlin, which is currently without effective treatment. Exosomes, efficient membranous nanoscale carriers of biological cargoes, could be useful. Experimental design: Myotube- and human serum-derived exosomes were investigated for their capabilities of restoring dysferlin protein and cellular functions in murine and human DYSF-/- cells. Moreover, dysferlinopathic patient serum- and urine-derived exosomes were assessed for their abilities as diagnostic tools for dysferlinopathy. Results: Here we show that exosomes from dysferlin-expressing myotubes carry abundant dysferlin and enable transfer of full-length dysferlin protein to DYSF-/- myotubes. Exogenous dysferlin correctly localizes on DYSF-/- myotube membranes, enabling membrane resealing in response to injury. Human serum exosomes also carry dysferlin protein and improve membrane repair capabilities of human DYSF-/- myotubes irrespective of mutations. Lack of dysferlin in dysferlinopathic patient serum and urine exosomes enables differentiation between healthy controls and dysferlinopathic patients. Conclusions: Our findings provide evidence that exosomes are efficient carriers of dysferlin and can be employed for the treatment and non-invasive diagnosis of dysferlinopathy.
Keywords: Dysferlinopathy; diagnostics; exosome; therapeutics.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

Similar articles
-
A promotive effect for halofuginone on membrane repair and synaptotagmin-7 levels in muscle cells of dysferlin-null mice.Hum Mol Genet. 2018 Aug 15;27(16):2817-2829. doi: 10.1093/hmg/ddy185. Hum Mol Genet. 2018. PMID: 29771357
-
[Ultrastructural changes of skeletal muscle tissue of patients with dysferlinopathy].Arkh Patol. 2025;87(1):28-36. doi: 10.17116/patol20258701128. Arkh Patol. 2025. PMID: 39943726 Russian.
-
Distinct effects of contraction-induced injury in vivo on four different murine models of dysferlinopathy.J Biomed Biotechnol. 2012;2012:134031. doi: 10.1155/2012/134031. Epub 2012 Feb 6. J Biomed Biotechnol. 2012. PMID: 22431915 Free PMC article.
-
Muscle Cells Fix Breaches by Orchestrating a Membrane Repair Ballet.J Neuromuscul Dis. 2018;5(1):21-28. doi: 10.3233/JND-170251. J Neuromuscul Dis. 2018. PMID: 29480214 Free PMC article. Review.
-
Dysferlin function in skeletal muscle: Possible pathological mechanisms and therapeutical targets in dysferlinopathies.Exp Neurol. 2016 Sep;283(Pt A):246-54. doi: 10.1016/j.expneurol.2016.06.026. Epub 2016 Jun 25. Exp Neurol. 2016. PMID: 27349407 Review.
Cited by
-
Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications.Int J Nanomedicine. 2020 Sep 22;15:6917-6934. doi: 10.2147/IJN.S264498. eCollection 2020. Int J Nanomedicine. 2020. PMID: 33061359 Free PMC article. Review.
-
Exosome-mediated delivery of an anti-angiogenic peptide inhibits pathological retinal angiogenesis.Theranostics. 2021 Mar 5;11(11):5107-5126. doi: 10.7150/thno.54755. eCollection 2021. Theranostics. 2021. PMID: 33859737 Free PMC article.
-
Engineered extracellular vesicles promote the repair of acute kidney injury by modulating regulatory T cells and the immune microenvironment.J Transl Med. 2025 Mar 10;23(1):304. doi: 10.1186/s12967-025-06268-x. J Transl Med. 2025. PMID: 40065372 Free PMC article.
-
The Functional Roles of RNAs Cargoes Released by Neutrophil-Derived Exosomes in Dermatomyositis.Front Pharmacol. 2021 Sep 17;12:727901. doi: 10.3389/fphar.2021.727901. eCollection 2021. Front Pharmacol. 2021. PMID: 34603043 Free PMC article.
-
Exosome-mediated improvement in membrane integrity and muscle function in dystrophic mice.Mol Ther. 2021 Apr 7;29(4):1459-1470. doi: 10.1016/j.ymthe.2020.12.018. Epub 2020 Dec 15. Mol Ther. 2021. PMID: 33333294 Free PMC article.
References
-
- Cohn RD, Campbell KP. Molecular basis of muscular dystrophies. Muscle Nerve. 2000;23:1456–71. - PubMed
-
- Cardenas AM, Gonzalez-Jamett AM, Cea LA, Bevilacqua JA, Caviedes P. Dysferlin function in skeletal muscle: Possible pathological mechanisms and therapeutical targets in dysferlinopathies. Exp Neurol. 2016;283:246–54. - PubMed
-
- Patel NJ, Van Dyke KW, Espinoza LR. Limb-girdle muscular dystrophy 2B and miyoshi presentations of dysferlinopathy. Am J Med Sci. 2017;353:484–91. - PubMed
-
- Cotta A, Carvalho E, da-Cunha-Junior AL, Paim JF, Navarro MM, Valicek J. et al. Common recessive limb girdle muscular dystrophies differential diagnosis: why and how? Arquivos de Neuro-psiquiatria. 2014;72:721–34. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources

