Naphthalene-based fluorescent probes for glutathione and their applications in living cells and patients with sepsis
- PMID: 29507630
- PMCID: PMC5835946
- DOI: 10.7150/thno.22252
Naphthalene-based fluorescent probes for glutathione and their applications in living cells and patients with sepsis
Erratum in
-
Erratum: Naphthalene-based fluorescent probes for glutathione and their applications in living cells and patients with sepsis: Erratum.Theranostics. 2024 Dec 1;14(19):7645. doi: 10.7150/thno.107439. eCollection 2024. Theranostics. 2024. PMID: 39659574 Free PMC article.
Abstract
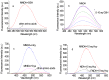
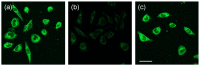
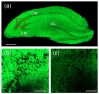
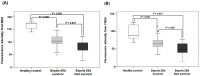
Rationale: Among the biothiols-related diseases, sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection and can result in severe oxidative stress and damage to multiple organs. In this study, we aimed to develop a fluorescence chemosensor that can both detect GSH and further predict sepsis. Methods: In this study, two new naphthalene dialdehyde compounds containing different functional groups were synthesized, and the sensing abilities of these compounds towards biothiols and its applications for prediction of sepsis were investigated. Results: Our study revealed that the newly developed probe 6-methoxynaphthalene-2, 3-dicarbaldehyde (MNDA) has two-photon is capable of detecting GSH in live cells with two-photon microscopy (TPM) under the excitation at a wavelength of 900 nm. Furthermore, two GSH detection probes naphthalene-2,3-dicarboxaldehyde (NDA) and 6-fluoronaphthalene-2,3-dicarbaldehyde (FNDA) not only can detect GSH in living cells, but also showed clinical significance for the diagnosis and prediction of mortality in patients with sepsis. Conclusions: These results open up a promising direction for further medical diagnostic techniques.
Keywords: GSH; Sepsis; cell imaging; diagnosis; fluorescence probe.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

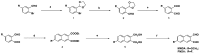




References
-
- Kim JS, Kwon WY, Suh GJ, Kim KS, Jung YS, Kim SH. et al. Plasma glutathione reductase activity and prognosis of septic shock. J Surg Res. 2016;200:298–307. - PubMed
-
- You KM, Kwon WY, Suh GJ, Kim KS, Kim JS, Bu J. et al. 975: PLASMA GLUTATHIONE REDUCTASE ACTIVITY IS ASSOCIATED WITH THE PROGNOSIS OF SEPTIC SHOCK. Crit Care Med. 2014;42:A1595.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

