Fluorogenic PNA probes
- PMID: 29507634
- PMCID: PMC5815273
- DOI: 10.3762/bjoc.14.17
Fluorogenic PNA probes
Abstract
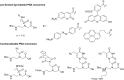
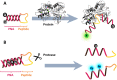
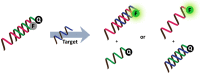
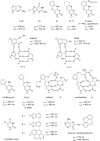
Fluorogenic oligonucleotide probes that can produce a change in fluorescence signal upon binding to specific biomolecular targets, including nucleic acids as well as non-nucleic acid targets, such as proteins and small molecules, have applications in various important areas. These include diagnostics, drug development and as tools for studying biomolecular interactions in situ and in real time. The probes usually consist of a labeled oligonucleotide strand as a recognition element together with a mechanism for signal transduction that can translate the binding event into a measurable signal. While a number of strategies have been developed for the signal transduction, relatively little attention has been paid to the recognition element. Peptide nucleic acids (PNA) are DNA mimics with several favorable properties making them a potential alternative to natural nucleic acids for the development of fluorogenic probes, including their very strong and specific recognition and excellent chemical and biological stabilities in addition to their ability to bind to structured nucleic acid targets. In addition, the uncharged backbone of PNA allows for other unique designs that cannot be performed with oligonucleotides or analogues with negatively-charged backbones. This review aims to introduce the principle, showcase state-of-the-art technologies and update recent developments in the areas of fluorogenic PNA probes during the past 20 years.
Keywords: DNA; RNA; fluorescence; molecular beacons; molecular probes; oligonucelotides.
Figures

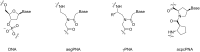
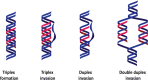
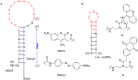

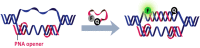












References
-
- Nielsen P E. Acc Chem Res. 1999;32:624–630. doi: 10.1021/ar980010t. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
