Recent advances on organic blue thermally activated delayed fluorescence (TADF) emitters for organic light-emitting diodes (OLEDs)
- PMID: 29507635
- PMCID: PMC5815274
- DOI: 10.3762/bjoc.14.18
Recent advances on organic blue thermally activated delayed fluorescence (TADF) emitters for organic light-emitting diodes (OLEDs)
Abstract
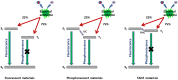
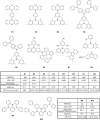
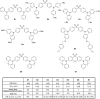
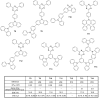
The design of highly emissive and stable blue emitters for organic light emitting diodes (OLEDs) is still a challenge, justifying the intense research activity of the scientific community in this field. Recently, a great deal of interest has been devoted to the elaboration of emitters exhibiting a thermally activated delayed fluorescence (TADF). By a specific molecular design consisting into a minimal overlap between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) due to a spatial separation of the electron-donating and the electron-releasing parts, luminescent materials exhibiting small S1-T1 energy splitting could be obtained, enabling to thermally upconvert the electrons from the triplet to the singlet excited states by reverse intersystem crossing (RISC). By harvesting both singlet and triplet excitons for light emission, OLEDs competing and sometimes overcoming the performance of phosphorescence-based OLEDs could be fabricated, justifying the interest for this new family of materials massively popularized by Chihaya Adachi since 2012. In this review, we proposed to focus on the recent advances in the molecular design of blue TADF emitters for OLEDs during the last few years.
Keywords: OLED; TADF; blue; electroluminescence; emitter.
Figures

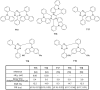
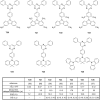
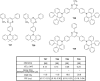




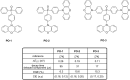
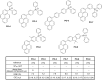

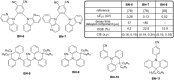

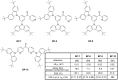
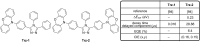
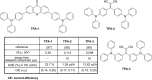
References
-
- Tang C W, VanSlyke S A. Appl Phys Lett. 1987;51:913–915. doi: 10.1063/1.98799. - DOI
-
- Gaspar D J, Polikarpov E. OLED Fundamentals: Materials, Devices, and Processing of Organic Light-Emitting Diodes. Boca Raton, FL, United States (US): CRC Press/Taylor and Francis Group; 2015.
-
- Fröbel M, Schwab T, Kliem M, Hofmann S, Leo K, Gather M C. Light: Sci Appl. 2015;4:e247. doi: 10.1038/lsa.2015.20. - DOI
-
- Baldo M A, O’Brien D F, Thompson M E, Forrest S R. Phys Rev B: Condens Matter Mater Phys. 1999;60:14422–14428. doi: 10.1103/PhysRevB.60.14422. - DOI
-
- Baldo M A, O’Brien D F, You Y, Shoustikov A, Sibley S, Thompson M E, Forrest S R. Nature. 1998;395:151–154. doi: 10.1038/25954. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
