Antioxidant and Cell-Signaling Functions of Hydrogen Sulfide in the Central Nervous System
- PMID: 29507650
- PMCID: PMC5817206
- DOI: 10.1155/2018/1873962
Antioxidant and Cell-Signaling Functions of Hydrogen Sulfide in the Central Nervous System
Abstract
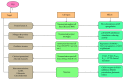
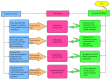
Hydrogen sulfide (H2S), a toxic gaseous molecule, plays a physiological role in regulating homeostasis and cell signaling. H2S is produced from cysteine by enzymes, such as cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE), cysteine aminotransferase (CAT), and 3-mercaptopyruvate sulfurtransferase (3MST). These enzymes regulate the overall production of H2S in the body. H2S has a cell-signaling function in the CNS and plays important roles in combating oxidative species such as reactive oxygen species (ROS) and reactive nitrogen species (RNS) in the body. H2S is crucial for maintaining balanced amounts of antioxidants to protect the body from oxidative stress, and appropriate amounts of H2S are required to protect the CNS in particular. The body regulates CBS, 3MST, and CSE levels in the CNS, and higher or lower levels of these enzymes cause various neurodegenerative diseases. This review discusses how H2S protects the CNS by acting as an antioxidant that reduces excessive amounts of ROS and RNS. Additionally, H2S regulates cell signaling to combat neuroinflammation and protect against central neurodegenerative diseases such as Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD), and amyotrophic lateral sclerosis (ALS).
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

